Introduction
Gastric and colorectal cancers rank among the top six most prevalent and lethal cancers globally1. In Colombia, by 2020, gastric cancer (GC) emerged as the third most common cancer following breast and lung cancers and was the foremost cause of cancer mortality. Colorectal cancer (CRC) positioned fourth in both incidence and mortality for the same period2.
The five-year relative survival rate for CRC stands at 65% overall, fluctuating from 72% to 91% in cases of cancer diagnosed with locoregional affliction, plummeting to 15% for those with advanced-stage disease3. In the United States, the median five-year survival rate for GC is recorded at 31%, and Europe reports a rate of 26%4. Notably, survival rates for localized disease have been documented to exceed 65%, highlighting the critical nature of early detection5.
It is well-established that endoscopic examinations equipped with biopsy capabilities serve as potent tools for the identification of preneoplastic and neoplastic lesions across both upper and lower sections of the gastrointestinal tract6,7. Through polypectomy, the overall incidence of mortality due to CRC can be nearly reduced by 30% (colon cancer: 29%-37%, rectal cancer: 27%, respectively)6.
Terms such as post-endoscopy or post-colonoscopy cancer, missed, or interval cancer are frequently employed to delineate cancers of the gastrointestinal tract diagnosed in the period between an endoscopically reported normal study and the next planned follow-up examination8-10.
A multitude of studies have pursued the cause behind this phenomenon. Identifying post-endoscopy CRC and GC is crucial as they are acknowledged as indicators of the quality of endoscopy11 and colonoscopy12 procedures. It has been determined that enhancing the quality of colonoscopy can either decrease the prevalence of cancer or facilitate earlier detection of the disease in over 50% of instances13. In 2014, Singh and colleagues contributed a meta-analysis outlining a 3.7% prevalence of post-colonoscopy colorectal cancer (PCCRC)14. Despite this, the exploration into the quality implications of upper digestive endoscopy remains scant. Studies have reported prevalences ranging from 25.8% to a mere 0.2% for post-endoscopy gastric cancer (PEGC); yet, detailed knowledge regarding its characteristics, incidence, and causal factors remains sparse15.
In drawing parallels between post-endoscopy colorectal and gastric cancers, this study invokes a method akin to that used by Plutarch16 in his seminal biographical work, Parallel Lives. Composed between 96 and 117 A.D., Plutarch’s narrative uniquely juxtaposes the lives of a Greek and a Roman figure, linked by a shared trait or dedication deemed defining by the author, ultimately comparing both individuals at the conclusion of their biographies. Similarly, our investigation seeks to parallel the narrative of post-endoscopy colorectal and gastric cancers by: (1) delineating the demographic, clinical, and endoscopic characteristics of two distinct patient cohorts, one diagnosed with PEGC and the other with PCCRC; (2) quantifying the incidence rates of PEGC and PCCRC; (3) juxtaposing the demographic, clinical, histological, and endoscopic findings across the studied groups, and (4) evaluating patient survival rates for PEGC and PCCRC, comparing these with the survival rates of other gastric and colorectal cancers identified without prior negative endoscopic evaluations for neoplasia.
Patients and methods
This research was conducted by gathering cases from the gastrointestinal oncology surgery departments of three quaternary care centers within the Medellín metropolitan area, Colombia, identifying instances of gastric and colorectal cancers that followed endoscopy and colonoscopy procedures between 2012 and 2021.
Data Collection and Management
Utilizing this dataset, a comparative analysis was performed between two patient cohorts managed ambispectively: one group with PEGC and another with PCCRC, covering the years 2012 to 2021. This analysis included patients diagnosed with gastric or colorectal cancer within a timeframe of six months to three years following an endoscopic procedure that did not initially detect neoplasia. The comparison focused on various dimensions including demographic (age, sex), clinical, and endoscopic characteristics, degree of differentiation, tumor stage (as determined by the tenth edition of the cTNM system of the American Joint Committee on Cancer), survival (calculated from the diagnosis of PEGC and PCCRC to the date of death), and potential reasons for the missed early detection (whether related to the quality of endoscopic or colonoscopic procedures or other factors).
Definitions
For patients with multiple endoscopies or colonoscopies, the analysis considered the most recent procedure. The interval defined for considering cancers as missed was set at 36 months following the last negative endoscopy or colonoscopy. The rate of missed cancers was determined by dividing the total number of missed cancers by the sum of missed cancers plus the total number of cancers diagnosed that met the inclusion criteria within the study period, in accordance with guidelines suggested by the World Endoscopy Organization (WEO).
The overall survival time was identified as the interval from the date of death, irrespective of cause, to the date of the histologically confirmed cancer diagnosis via endoscopy or colonoscopy, based on the reports from the procedures; essentially, this pertains to the second endoscopy or colonoscopy for cases of PEGC or PCCRC.
Inclusion and Exclusion Criteria
The principal criterion for inclusion in this study was the identification of patients who received a histopathological diagnosis of either gastric or colorectal adenocarcinoma within the study’s timeframe, with a requisite follow-up period of at least one year. We also included patients who had undergone previous surgeries, such as colectomies or gastrectomies, as well as those diagnosed with CRC or GC at an external facility before being referred to the participating study institutions for treatment. Patients were excluded if their gastric (six patients) or colorectal (four patients) cancers were diagnosed via imaging techniques, if they had a history of familial adenomatous polyposis syndrome, which invariably leads to CRC (three patients), or if they suffered from inflammatory bowel disease, including Crohn’s disease (two patients) and ulcerative colitis (nine patients).
Statistical Analysis
For continuous variables, we calculated means, standard deviations, medians, and ranges. Categorical data were expressed as frequency counts and percentages. We computed 95% confidence intervals (CIs) for proportions using Wilson’s method. The analysis employed parametric methods for normally distributed continuous data (t-tests) and non-parametric methods (Mann-Whitney U test) for data not normally distributed.
Chi-square and Fisher’s exact tests were applied to categorical data. To reduce the risk of type I errors, only variables previously identified as risk factors for interval gastric cancer (IGC) or those with a plausible pathophysiological connection to IGC were included in our analysis.
We estimated one- and two-year survival probabilities for IGC and non-interval gastric adenocarcinoma using the Kaplan-Meier method. The Log-Rank test was utilized to compare differences in overall survival. All statistical analyses were two-tailed, with p-values below 0.05 deemed statistically significant. Statistical computations were performed using IBM SPSS software, version 24, at the sponsoring institution.
Ethical Considerations
The procedures were conducted in strict accordance with the ethical standards set by the responsible committee on human experimentation (both institutional and national) and aligned with the principles of the 1964 Declaration of Helsinki and its subsequent amendments. We ensured the protection of data confidentiality. It is important to note that this article does not contain any personal information that could lead to the identification of patients involved. This research compared data from two previously analyzed studies, which were obtained from a secondary source without any direct intervention on the patients, hence, informed consent was not required.
Results
The study compared a total of 128 patients across two cohorts: 66 patients with PEGC, which translates to a rate of 7.3% of the patients evaluated (828 gastric cancer patients over a ten-year period) and 68 patients with PCCRC, representing a rate of 6.9% of the evaluated CRC patients (919 colorectal cancer patients over the same ten-year span) (Figure 1).
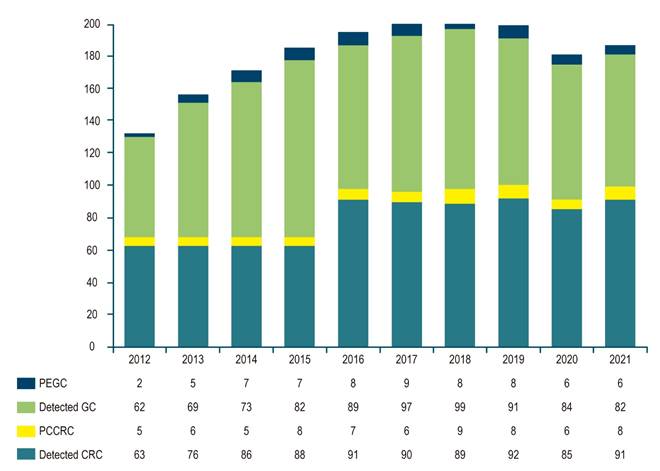
Author’s own research.
Figure 1 Number of GC/PEGC and CRC/PCCRC Cases Detected between 2012 and 2021.
Demographic Characteristics
The median age in the group with PCCRC was 74 years, whereas it was 66 years for the group with PEGC. Those with cancer diagnosed after colonoscopy exhibited a median age that was eight years greater than that of the post-endoscopy cancer group (74 versus 66 years), which was a significant disparity as per the Student’s t-test (p = 0.0012).
The sex distribution exhibited a predominance of males in the PEGC group (69% compared to 31%), while in the PCCRC group, the distribution by sex showed no marked differences (53% male versus 47% female). The male predominance historically seen in gastric cancer (2:1 ratio) persists. The characteristics of the two patient groups are detailed in Table 1.
Table 1 Characteristics of Patients with PEGC and PCCRC
| Characteristic | PEGC (n = 66) Rate: 7.3% (%) | PCCRC (n = 68) Rate: 6.9% (%) | p-Value | |
|---|---|---|---|---|
| Post-endoscopy/Post-colonoscopy Cancer | Yes | 66 | 68 | 0.591 |
| No | 762 | 851 | ||
| Average Age (Years) | 66 ± 16.6 | 74 ± 8.6 | 0.001 | |
| Age Group | < 55 | 13 (19) | 9 (13) | 0.344 |
| 55-65 | 14 (21) | 10 (15) | ||
| 65-75 | 28 (43) | 28 (41) | ||
| > 75 | 11 (17) | 21 (31) | ||
| Sex | Male | 46 (69) | 36 (53) | 0.09 |
| Female | 20 (31) | 32 (47) | ||
| Screening Study | Yes | 30 (46) | 18 (26) | 0.004 |
| No | 36 (54) | 50 (74) | ||
| Premalignant Lesions | Yes | 32 (48) | 25 (37) | 0.260 |
| No | 34 (52) | 43 (63) | ||
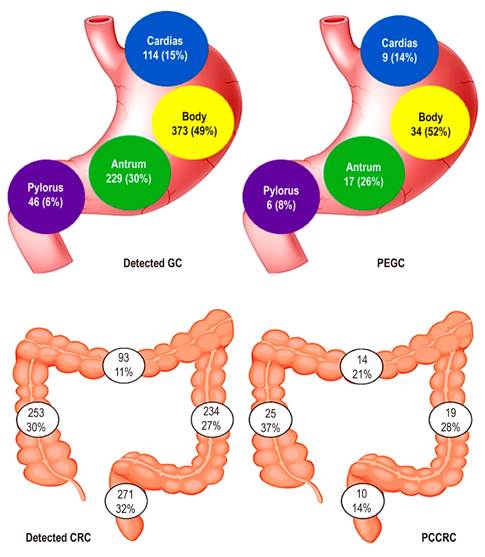
| Location | Cardias-Fundus: 9 (14) | Ascending Colon: 25 (37) | 0.002 | |
| Body: 34 (52) | Transverse: 14 (21) | |||
| Antrum: 17 (26) | Descending Colon: 19 (28) | |||
| Pylorus: 6 (8) | Rectum: 10 (14) | |||
| Differentiation | Well | 28 (42) | 50 (74) | 0.001 |
| Poor | 38 (58) | 18 (26) | ||
| Early Cancer | Yes | 14 (21) | 10 (15) | 0.768 |
| No | 52 (79) | 58 (85) | ||
| Stage | 0/I | 14 (21) | 10 (15) | < 0.01 |
| II | 13 (19) | 25 (37) | ||
| III | 16 (24) | 30 (44) | ||
| IV | 23 (36) | 3 (4) | ||
Author’s own research.
Clinical Characteristics
Within the cohort of patients with PEGC, the impetus for endoscopy was more often due to alarm symptoms such as dysphagia, hematemesis, melena, vomiting, and constitutional syndrome in 54% of cases. Conversely, for the PCCRC group, the trigger for colonoscopy was largely the presence of alarming symptoms like rectal bleeding, anemia, abdominal pain, or changes in bowel habits, reported in 74% of cases, marking a significant difference (p = 0.004). Screening or early detection studies were conducted more commonly in the PEGC cohort (46%) compared to the PCCRC patients (26%).
Endoscopic Characteristics
The presence of concurrent premalignant lesions was noted to be more prominent in the PEGC group (48%) than in the PCCRC group (37%), yet this difference did not reach statistical significance (p = 0.260).
The median time between a negative esophagogastroduodenoscopy and the diagnosis of IGC was 14.4 months (range: 2-46 months). The most frequently encountered findings in the negative esophagogastroduodenoscopy were gastritis (73%), intestinal metaplasia (36%), gastric atrophy (45%), and gastric ulcer (29%). Two percent had an esophagogastroduodenoscopy reported as normal. Meanwhile, the median time span from a negative colonoscopy to the diagnosis of PCCRC was 22.8 months, with diverticulosis being more prevalent in this group (41.2%) than in those with cancer identified on the initial colonoscopy.
Regarding the location of lesions missed during upper gastrointestinal evaluation, no significant differences were observed across the sites of the cardias/fundus, body, antrum, and pylorus (p = 0.925). However, a significant proximal involvement (right colon and transverse colon) was demonstrated in the PCCRC group (p = 0.006) as shown in Figure 2.
Histological Characteristics
In the cohort with PEGC, the histological differentiation most commonly observed was of the poorly differentiated variety (58%), whereas in the PCCRC group, this was seen less frequently (26%), a difference that proved to be statistically significant (p = 0.001). No significant differences were noted in the occurrence of early-stage GC or CRC (21% compared to 19%, p = 0.768).
Tumor Staging
It was observed in the PEGC group that 21% of patients were in early tumor stages (stages 0 and I) as opposed to 15% in the PCCRC cohort. A higher incidence of advanced tumor stages was found in the PEGC patients (36%) compared to those in the PCCRC cohort (4%) (p < 0.001).
Causal Analysis According to the WEO for PCCRC
In evaluating post-colonoscopy colorectal cancer, 62 of the 68 affected patients were assessed on this issue. It was discovered that 61.3% of the cases occurred as a consequence of inadequately performed colonoscopies (Table 2).
Table 2 Causal Analysis of PCCRC According to the WEO
| Characteristic | PCCRC Patients (%) |
|---|---|
| n | 68 |
| Missed lesion, with prior adequate colonoscopy | 18 (29) |
| Missed lesion, with prior inadequate colonoscopy | 38 (61.3) |
| Detected lesions not resected | 0 (0) |
| Incomplete resection of previously identified lesion | 6 (9.7) |
Author’s own research.
Survival Analysis
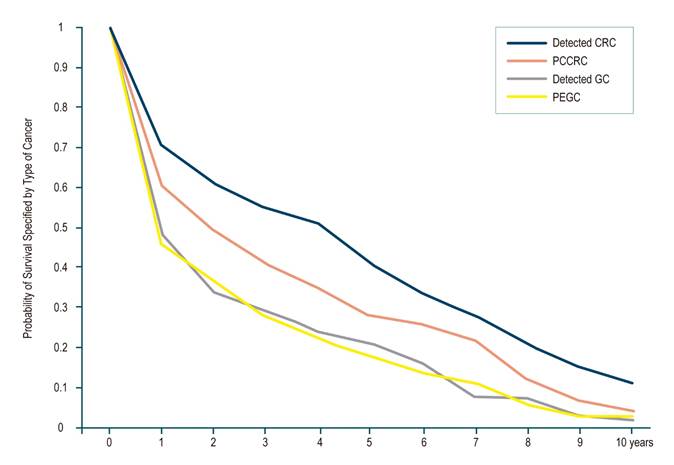
Kaplan-Meier survival curves for patients with PEGC and PCCRC are depicted in Figure 3. Notably, 72% of these patients had undergone a negative high gastrointestinal endoscopy less than two years prior to their GC diagnosis.
In terms of specific cancer survival rates for PCCRC, there was a 73.4% survival at one year (95% confidence interval [CI]: 72.2%-74.7%), 61% at three years (CI 95%: 56.3%-65.4%), 58% at five years (CI 95%: 54%-62%), and 55.2% at ten years (CI 95%: 51%-60%). These rates were lower compared to CRC detected during screening; 83.0% survival at one year (CI 95%: 82.3%-85%), 71.5% at three years (CI 95%: 70.3%-72.5%), 67% at five years (CI 95%: 65.6%-67.6%), and 63.0% at ten years (CI 95%: 62.0%-65.3%). The survival for PEGC was assessed at one and two years and revealed no statistically significant differences when compared with detected GC, as shown in Table 3.
Table 3 Comparative Survival between Unnoticed GC and Detected GC
| 1-Year Survival (%) | 2-Year Survival (%) | Median Survival (Months) | |
|---|---|---|---|
| PEGC | 45.7% (36.9%-61.3%) | 37.2% (30.3%-37.6%) | 12 (5.7-26) |
| Detected GC | 48.4% (44.1%-53.4%) | 34.4% (24.1%-47.7%) | 13 (11.2-15.7) |
| Overall | 49.8% (45.1%-53.7%) | 36.7% (33.8%-38.2%) | 12.8 (6.1-13.7) |
Author’s own research.
Discussion
Upper endoscopy and colonoscopy play a crucial role in the diagnosis, prevention, and management of GC and CRC. However, the last decade has seen mounting evidence of a significant rate of false negatives or missed detections in both procedures, as population studies have suggested17,18. The risk of missing cancers, a concern for the past and looking ahead, is a prevailing thought among clinicians and patients alike19. Interestingly, rates of missed diagnoses during upper endoscopy have not been scrutinized to the same degree as colonoscopy, and it was only two decades ago that the first study addressing missed upper gastrointestinal cancers in Western populations emerged20.
This study stands as the inaugural research within our nation to evaluate and contrast the missed or interval rates for GC and CRC following endoscopy and colonoscopy. It uses two patient cohorts from oncology centers with established diagnoses of GC and CRC for reference. The missed rate of 7.3% found for PEGC, as per WEO guidelines, falls within the range cited in international literature (4.6% to 14.3%) but exceeds the preferred threshold of less than 5%21. PCCRC rate in our research was found to be 6.9%, surpassing the rates in Korea (0.1%)22, Turkey (1.8%)23, and Portugal (3.8%)24, yet aligning with figures from Sweden (7.9%)25, Hong Kong (7.9%)26, the United Kingdom (7.4%)27, and Belgium (7.6%)28, and remaining below those reported in Denmark (8.6%)29, the United States (9.4%)30, and Israel (12.8%)31.
Notably, there is a lack of a standardized definition for post-endoscopy or post-colonoscopy GC or CRC, which results in significant discrepancies among researchers, especially concerning the time frame between the two endoscopic studies (ranging from three to fives years). Furthermore, there is no consensus on the calculation method, whether as a rate, index, or percentage32. The WEO released a consensus in 2018 on post-colonoscopy and post-imaging colorectal cancer33 to standardize terminology, identification, analysis, and reporting of these cases. They recommend the term “post-colonoscopy colorectal cancer” for cancers identified following a colonoscopy that failed to diagnose cancer, further dividing these into interval cancers (when cancer is found before the recommended date for the next screening or surveillance exam) and non-interval cancers.
In our series, PEGC did not exhibit a preferential anatomical location, contrasting with findings of higher frequency in the post-surgical stomach10. Unlike PEGC, the right-sided location of lesions in PCCRC has been identified as a risk factor for post-colonoscopy cancer. Our study pinpointed a greater incidence of post-colonoscopy colon cancer in the right colon, consistent with previous studies25,34-36. This could be attributed to challenges such as incomplete procedures or poor bowel preparation since the right colon is more difficult to clean with oral agents, offers less clear landmarks, and is technically more demanding in reaching the proximal colon and maintaining appropriate positions for polypectomies. Additionally, a connection was observed between post-colonoscopy colorectal cancer and prior diverticulosis diagnoses, corroborating literature reports25,34. The presence of diverticular disease complicates colonoscopy due to increased patient discomfort, altered mucosa in the affected zones, and the hazard of mistaking neoplastic tissue for diverticular inflammation37. These insights should heighten colonoscopists’ vigilance during procedures in high-risk individuals (those with inflammatory bowel disease or adenomatous polyposis syndromes) and in assessments of the right colon. Should there be any doubts about the thorough examination of an intestinal segment, or if a deep cecal intubation is not achieved, it is prudent to recommend repeating the study or opting for an alternative method to exclude unobserved neoplasms from the initial examination.
The discovery and comparison of rates for PEGC and PCCRC are intended to spotlight a concern that might otherwise fly under the radar for many endoscopists and endoscopy services. Awareness of post-endoscopy and post-colonoscopy cancer in daily practice is limited, largely because it is a relatively rare occurrence. Moreover, linking cancer to a prior endoscopy or colonoscopy is challenging, as these events can be separated by months or even years. Often, endoscopists or colonoscopists are unaware of a cancer that emerges several years after the index study. There might also be a degree of complacency, a belief that a cancer found after endoscopy or colonoscopy is a fast-growing tumor rather than an overlooked or incompletely resected lesion, or that missing lesions are an issue that affects other colleagues or are too rare to warrant concern. From a patient’s standpoint, however, it can make a significant difference-it can mean the difference between having cancer or not, receiving treatment for an advanced-stage tumor, or facing a worse prognosis due to delayed diagnosis. Additionally, delayed diagnoses incur extra costs to the healthcare system.
It is widely acknowledged that most post-endoscopy or post-colonoscopy cancers are preventable (at least half of them)38. Research has indicated a more than tenfold variation in PCCRC rates among colonoscopists. Physicians with lower rates of colonoscopy performance, polyp detection, and polyp resection are more likely to encounter PCCRC28.
This study contributes several key insights: firstly, that reducing PEGC and PCCRC rates could lower the incidence of GC and CRC and improve patient prognosis; secondly, it corroborates existing literature that high-quality endoscopy or colonoscopy will decrease PEGC and PCCRC rates11,39; and thirdly, it provides clinical evidence on the characteristics of these overlooked neoplasms. For instance, PCCRC is typically found in a proximal location, affects older patients, and impacts survival, whereas PEGC patients tend to be younger, have more undifferentiated tumors, and present in more advanced stages.
Technological advancements in endoscopy equipment, such as high-definition endoscopes, and techniques like narrow-band imaging (NBI) and chromoendoscopy, have gradually reduced the rate of overlooked gastrointestinal cancers40. An emerging element in this landscape is the supportive diagnostic role of artificial intelligence (AI). Recent incorporation of AI in colonoscopy has halved the risk of missing colorectal neoplasms (compared to standard colonoscopy), especially reducing the oversight of flat neoplasms under 10 mm in both the proximal and distal colon, and potentially enhancing early-stage neoplasm detection41,42.
Conclusions
The rates of missed cancers following endoscopy or colonoscopy stand at 7.3% and 6.9%, respectively. Distinctions between PEGC and PCCRC include differences in the reasons for study referrals, with PCCRC presenting more symptoms, more proximal lesion locations, a greater degree of tumor undifferentiation, and more advanced tumor stages in PEGC, along with poorer survival outcomes for PCCRC. Upon acknowledging these rates, it is crucial to implement strategies by various associations to strive for the goal set by the WEO of maintaining these rates below 5%.











 texto em
texto em