Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Química
Print version ISSN 0120-2804
Rev.Colomb.Quim. vol.42 no.1 Bogotá Jan./Apr. 2013
Synthesis of new N-phenyl-N-(1-phenylhex- 5-en-1-yl)acetamides and their 1H-NMR conformational study
Síntesis de nuevas N-fenil- N-(1-fenilhex-5-en-1-il) acetamidas y su estudio conformacional mediante 1H-RMN
Síntese de novas N-fenil- N-(1-fenilhex-5-en-1-il) acetamidas e o seu estudo conformacional por 1H-RMN
Mauricio Acelas1, Elizabeth Gil2, Markus Doerr3, Martha C. Daza3, Juan-Manuel Urbina1
1 Laboratorio de Química Orgánica y Biomolecular - LQOBio, Universidad Industrial de Santander, Ciudad Universitaria, AA 678, Bucaramanga, Colombia.
2 Facultad de Ciencias, Pontificia Universidad Javeriana, Carrera 7 Nº 43-82, Bogotá DC. Colombia.
3 Grupo de Bioquímica Teórica-GBQT, Universidad Industrial de Santander, Ciudad Universitaria, AA 678, Bucaramanga, Colombia.
* Autor para correspondencia: jurbina@uis.edu.co
Recibido: 14 de enero de 2013 • Aceptado: 22 de abril de 2013
Abstract
Antifungal and antiparasitic activities for N-acetyl derivatives of different N-(prop)butenylamines have been previously evaluated and reported. Consequently, an efficient and versatile synthesis procedure and complete characterization of different N-phenyl-N-(1-phenylhex-5-en-1-yl)acetamides is presented. Two conformational isomers were observed for one of the compounds in their 1H/13C-NMR spectra. The conformational analysis was carried out using the B3LYP functional with the 6-31+G(2d,p) basis and the NMR spectroscopic data. The dihedral angle values of the allylic system obtained by both computational methods and 1H-NMR data analysis (Garbisch's equation) were compared and used to successfully determine the conformational structures and the intramolecular interaction responsible for signal duplicity and chemical shifting respectively.
Keywords: Acetamides, conformational analysis, computational calculation, NMR, Garbisch equation.
Resumen
Previamente se han evaluado y reportado las propiedades antifúngicas y antiparasitarias para derivados de N-acetil procedentes de diferentes N-(prop)butenilaminas. En este sentido, la esta investigación presenta un procedimiento de síntesis versátil y eficiente, con la caracterización completa de diferentes N-fenil-N-(1-fenilhex-5- en-1-yl)acetamidas. Se observaron dos isómeros conformacionales para uno de los compuestos en su espectro 1H/13CRMN. El análisis conformacional se llevó a cabo usando B3LYP funcional con base en 6-31+G(2d,p) y los datos de espectroscopia RMN. Los valores de ángulo dihedro del sistema alílico —obtenidos por los métodos computacionales citados y el análisis de los datos derivados de 1H-RMN usando la ecuación de Garbisch—, se compararon y se usaron para determinar exitosamente las estructuras conformacionales isoméricas y la interacción intramolecular responsables de la duplicidad de señales y del desplazamiento químico, respectivamente.
Palabras clave: acetamidas, isómeros conformacionales, cálculos computacionales, RMN, ecuación de Garbisch.
Resumo
As atividades antifúngicas e antiparasitárias de N-acetil derivados de diferentes N-(prop)butenilaminas têm sido previamente avaliadas e relatadas. Neste trabalho, apresenta-se uma rota de síntese eficiente e a caracterização completa de diferentes N-fenil-N-(1-fenilhex-5-en-1-il)acetamidas. Nos espectros 1H/13C-RMN foram observados dois isômeros conformacionais para um dos compostos. Foi feita uma análise conformacional usando o funcional B3LYP com bases 6-31+G(2d,p) e os dados de 1H-RMN. Os valores dos ângulos diedros do sistema alílico obtidos por métodos computacionais e por análise de dados de 1H-RMN (equação de Garbish) foram comparados e usados para determinar as estruturas dos confôrmeros, o que permitiu determinar as interações intramoleculares responsáveis do diferente deslocamento químico e a conseqüente duplicidade dos sinais no composto que apresentou os dois confôrmeros.
Palavras-chave: acetamidas, isómeros conformacionais, cálculo computacional, RMN, equação de Garbisch.
Introduction
Target oriented synthesis (TOS) is one of the most important methodologies in organic chemistry to access biologically active compounds (1). Molecules including quinoline and tetrahidroquinoline derivatives are widely known for their biological and pharmacological activity as well as for their uses in organic electronics (2-3). Previous reports from our research group have shown both antifungal and antiparasitic activities of N-phenyl-α-2-propen-1-yl benzenpropanamines 1a-e (4-5). The use of 1a as synthon for different N-heterocycles containing the tetrahydrolepidine and quinoline moiety is well known. These substances are similar to isolated compounds from Galipea longiflora and Galipea officinalis, which have been studied and used against fever, dysentery, malaria and leishmaniasis treatment, among others (6-13). Literature reports have shown that acetylation of N-(prop)butenylamines turns these compounds into biologically active N-(prop)butenylacetamides (4,14-15).
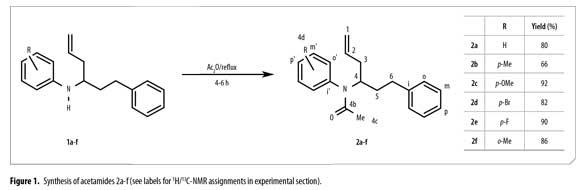
Quantum chemical methods are nowadays valuable tools in chemistry. Typical applications include calculations of molecular properties, either in order to interpret experimental data or to predict properties of new molecules, and the investigation of reaction mechanisms. Characterization of favoured conformational structures for different biologically active compounds using quantum chemical calculations and NMR (Nuclear Magnetic Resonance) data analysis has also been reported. (16-21). Keeping in view the above facts, new N-phenyl-N-(1-phenylhex-5-en-1-yl)acetamides 2a-f were prepared by N-acetylation of compounds 1a-f. Their synthesis and characterization data is presented. Quantum chemical calculations were performed in order to interpret the experimental 1H/13C-NMR data and to obtain information about the conformational structure of the synthesized compounds. Biological activity of compounds 2a-f is currently under study.
Materials and methods(Experimental section)
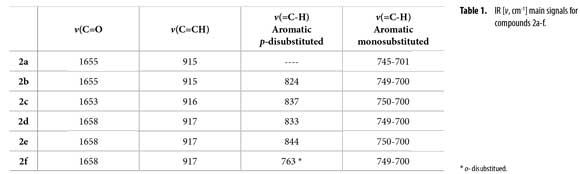
IR spectra were obtained on a FT-IR Bruker Tensor 27™, using KBr windows. IR main signals are condensed in Table 1. GC/MS data were acquired on a HP5890A Series II™ gas chromatography equipped with a HP-5MS™ column (5% methyl phenyl siloxane, 30 m x 0.25 mm x 0.25 µm) and a selective mass detector HP5972™ (EI, 70 eV). NMR spectra were recorded on a Bruker Avance-400™. Coupling constants J are reported in Hertz. See Figure 1 for 1H/13C assignment.
General procedure for the synthesis of N-phenyl-N-(1-phenylhex-5-en-1yl)acetamides 2a-f
A round bottom flask with a reflux condenser, a thermometer and a magnetic stirrer was filled with 0.35 g (1.24 mmol) of N-phenyl-α-2-propen-1-ylbenzenpropanamine (previously prepared) (3) and 3.80 g (37.02 mmol) of acetic anhydride. The reaction mixture was refluxed for 4-6 h and neutralized using NaHCO3. NaOH 0.1 M was used to adjust the mixture to pH 12. Ethyl acetate was used for extraction (20 mL x 3). The organic layer was dried over Na2SO4, the solvent removed and the crude product purified by column chromatography on SiO2 using n-heptane and ethyl acetate gradient mixtures. Compounds characterization was carried out using IR, 1H, 13C NMR and GC-MS. Compounds purity was monitored by GC-FID and it was higher than 98%.
Synthesis of N-phenyl-N-(1-phenylhex-5-en-1-yl)acetamide 2a
From N-phenyl-α-2-propen-1-ylbenzen propanamine 1a (0.33 g, 1.31 mmol) and acetic anhydride (4.32 g, 42.30 mmol). Pure compound 2a was obtained after column chromatography as a brownish oil. Rf = 0.50 (n-heptane:AcOEt, 5:2). MS [EI, 70 eV] (m/z, %): 293 (M+·,2), 252 (38), 210 (100), 117 (13), 91 (47). δH ppm (CDCl3, 400 MHz): 1.62 (td, 3J= 8.0, 7.3, 2H5), 1.72 (s, 3H4c), 2.03-2.19 (m, 2H3), 2.63 (ddd, 2J= 14.4; 3J= 8.3, 7.6, 1H6), 2.71 (ddd, 2J=14.4; 3J= 8.3, 7.6, 1H6), 4.90-5.05 (m, 3H1,4), 5.76 (dddd, 3J= 17.7, 9.5, 6.8, 6.4, 1H2), 6.94-7.39 (m, 10HArom). δC ppm (CDCl3, 100 MHz): 23.63(4c), 33.13(6), 34.57(5), 37.75(3), 54.07(4), 117.11(1), 125.86(p), 128.25(2xm), 128.36(2xo), 128.74(p'), 129.36(2xm'), 129.86(2xo'), 135.66(2), 139.37(i'), 141.81(i), 171.03(4b).
Synthesis of N-Phenyl-N-(4-methylphenylhex-5-en-1-yl)acetamide 2b
From of N-(4-methylphenyl)-α-2-propen-1-ylbenzenpropanamine 1b (1.07 g, 4.03 mmol) and acetic anhydride (10.79 g, 105.75 mmol). Pure compound was obtained after chromatography column as a brownish oil. Rf = 0.50 (n-heptane:AcOEt, 5:2). MS [EI, 70 eV] (m/z, %): 307 (M+·,2), 266 (37), 224 (100), 150 (11), 91 (40). δH ppm (CDCl3, 400 MHz): 1.68 (td, 3J= 8.1, 7.3, 2H5), 1.79 (s, 3H4c), 2.08-2.29 (m, 2H3), 2.39 (s, 3H4d), 2.70 (ddd, 2J= 14.5; 3J= 8.1, 7.5, 1H6), 2.77 (ddd, 2J= 14.5; 3J= 8.1, 7.5, 1H6), 4.93-5.18 (m, 3H1,4), 5.83 (dddd, 3J= 17.5, 9.3, 6.9, 6.5, 1H2), 7.06 (d, 3J= 8.2, 2Ho'), 7.14-7.33 (m, 7HArom). δC ppm (CDCl3, 100 MHz): 20.98(4d), 23.47(4c), 33.04(6), 34.46(5), 37.69(3), 53.80(4), 116.95(1), 125.75(p), 128.17(2xm), 128.27(2xo), 129.51(2xo'), 129.90(2xm'), 135.64(2), 136.49(p'), 138.18(i'), 141.79(i), 171.16(4b).
Synthesis of N-Phenyl-N-(4-methoxyphenylhex-5-en-1-yl)acetamide 2c
From N-(4-methoxyphenyl)-α-2-propen-1-ylbenzenpropanamine 1c (0.35 g, 1.24 mmol) and acetic anhydride (3.67 g, 35.96 mmol). Pure product was obtained after chromatography column as a brownish oil. Rf = 0.40 (n-heptane:AcOEt, 5:2). MS [EI, 70 eV] (m/z, %): 323 (M+·, 2), 282 (28), 240 (100), 91 (51). δH ppm (CDCl3, 400 MHz): 1.67 (td, 3J= 8.1, 7.3, 2H5), 1.80 (s, 3H4c), 2.06-2.30 (m, 2H3), 2.70 (ddd, 2J= 14.4; 3J= 8.3, 7.6, 1H6), 2.77 (ddd, 2J=14.4; 3J= 8.3, 7.6, 1H6), 3.83 (s, 3H4d), 4.98-5.14 (m, 3H1,4), 5.83 (dddd, 3J=17.6, 9.5, 6.8, 6.5, 1H2), 6.92 (d, 3J= 9.2, 2Ho'), 7.04-7.35 (m, 7HArom). δC ppm (CDCl3, 100 MHz): 23.55(4c), 33.12(6), 34.54(5), 37.72(3), 53.78(4), 55.43(4d), 114.43(2xm'), 117.06(1), 125.87(p), 128.27(2xm), 128.39(2xo), 130.87(2xo'), 131.75(i'), 135.76(2), 141.88(i), 159.20(p'), 171.57(4b).
Synthesis of N-Phenyl-N-(4-bromophenylhex-5-en-1-yl) acetamide 2d
From N-(4-bromophenyl)-α-2-propen-1-ylbenzenpropanamine 1d (0.68 g, 2.06 mmol) and acetic anhydride (7.34 g, 71.91 mmol). Pure product was obtained after column chromatography as a brownish oil. Rf = 0.47 (n-heptane:AcOEt, 5:2). MS [EI, 70 eV] (m/z, %): 373 (M+·,2), 330 (33), 290 (95), 288 (100), 91 (82). δH ppm (CDCl3, 400 MHz): 1.61-1.73 (m, 2H5), 1.79 (s, 3H4c), 2.17 (ta, 3J= 7.0, 2H3), 2.65-2.79 (m, 2H6),4.94-5.13 (m, 3H4,1), 5.81 (dddd, 3J=17.2, 10.1, 6.6, 6.3, 1H2), 7.06 (d, 3J= 8.8, 2Ho'), 7.14-7.31 (m, 5HArom), 7.55 (d, 3J= 8.8, 2Hm'). δC ppm (CDCl3, 100 MHz): 23.63(4c), 33.07(6), 34.59(5), 37.55(3), 54.07(4), 117.31(1), 122.39(p'), 125.95(p), 128.21(2xm), 128.41(2xo), 131.55(2xo'), 132.62(2xm'), 135.44(2), 138.42(i'), 141.53(i), 170.67(4b).
Synthesis of N-Phenyl-N-(4-fluorophenylhex-5-en-1-yl)acetamide 2eFrom N-(4-fluorophenyl)-α-2-propen-1-ylbenzenpropanamine 1e (0.63 g, 2.34 mmol) and acetic anhydride (6.80 g, 66.62 mmol). Pure product was obtained after column chromatography as a brownish oil. Rf = 0.43 (n-heptane:AcOEt, 5:2). MS [EI, 70 eV] (m/z, %): 311 (M+·,2), 270 (36), 228 (100), 117 (15), 91 (51). δH ppm (CDCl3, 400 MHz): 1.71-1.82 (m, 2H5), 1.87 (s, 3H4c), 2.22-2.28 (m, 2H3), 2.79 (ddd, 2J= 13.8; 3J= 9.6, 6.9, 1H6), 2.84 (ddd, 2J= 13.8; 3J= 9.6, 6.9, 1H6), 5.06-5.22 (m, 3H1,4), 5.91 (dddd, 3J= 17.0, 10.6, 6.6, 6.3, 1H2), 7.16-7.40 (m, 9HArom). δC ppm (CDCl3, 100 MHz): 23.55(4c), 33.03(6), 34.53(5), 37.52(3), 53.89(4), 116.28(2xm') (d, 2J= 22.5), 117.21(1), 125.91(p), 128.18(2xm), 128.38(2xo), 131.55(2xo'), 135.19(i') (d, 4J= 3.4), 135.49(2), 141.57(i) 162.07(p') (d, 1J=249.1), 171.02(4b).
Synthesis of N-Phenyl-N-(2-methylphenylhex-5-en-1-yl)acetamide 2f
From N-(2-methylphenyl)-α-2-propen-1-ylbenzen propanamine 1f (0.67 g, 2.52 mmol) and acetic anhydride (7.23 g, 70.81 mmol). Pure product was obtained after column chromatography as a brownish oil. Rf = 0.53 (n-heptane:AcOEt, 5:2). GC showed a single signal. MS [EI, 70 eV] (m/z, %): 307 (M+·, 2), 266 (39), 224 (100), 118 (13), 91 (51). NMR data for α conformer: δH ppm (CDCl3, 400 MHz): 1.73 (s, 3H4c), 1.74-1.82 (m, 2H5), 2.26 (s, 3H4d), 2.45-2.51; 2.52-2.59 (m, 2H3), 2.59-2.67 (m, 2H6), 4.75-4.86 (m, 1H4), 5.08-5.20 (m, 2H1), 5.89 (dddd, 3J= 17.3, 9.9, 6.7, 6.5, 1H2), 7.07-7.34 (m, 9HArom). δC ppm (CDCl3, 100 MHz): 18.44(4d), 23.15(4c), 33.45(6), 33.57(5), 38.23(3), 55.87(4), 117.08(1), 125.81(p), 126.87(m'), 128.21(p'), 128.25(2xo), 128.32(2xm), 129.64(o'), 131.54(m''), 135.93(2), 136.87(i'), 139.35(o''), 141.67(i), 171.06(4b). NMR data for β conformer: δH ppm (CDCl3, 400 MHz): 1.49-1.61 (m, 2H5), 1.77 (s, 3H4c), 1.86-2.07; 2.36-2.48 (m, 2H3), 2.27 (s, 3H4d), 2.77 (ta, 3J= 8.4, 2H6), 4.75-4.86 (m, 1H4), 4.95-5.01 (m, 2H1), 5.69 (dddd, 3J= 16.6, 10.8, 7.9, 6.0, 1H2), 7.07-7.34 (m, 9HArom). δC ppm (CDCl3, 100 MHz): 18.49(4d), 23.06(4c), 33.14(6), 33.31(5), 36.61(3), 55.83(4), 117.18(1), 125.81(p), 126.95(m'), 128.17(p'), 128.24(2xo), 128.34(2xm), 129.32(o'), 131.60(m''), 135.42(2), 137.02(i'), 139.22(o''), 141.97(i), 171.04(4b).
Conformational analysis
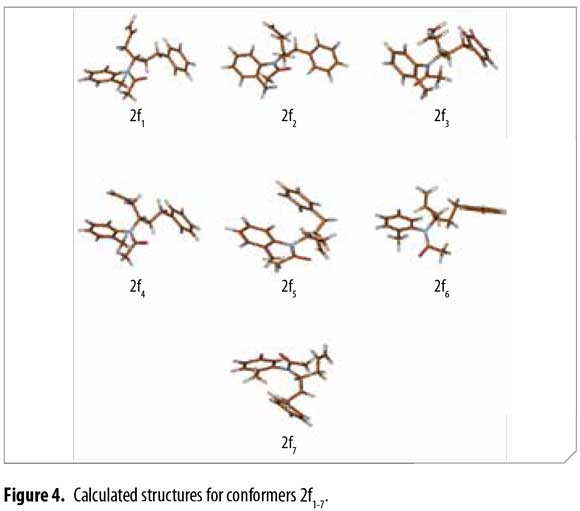
Ten chemically reasonable structures for compound 2f were used as the starting point for energy minimization using the Parameterized Model 3 (PM3) semi-empirical method (22). Geometry optimization and vibrational frequency calculations (for verifying that the structures correspond to minima on the potential energy surface) were then carried out using the B3LYP functional with the 6-31+G(2d,p) basis set as implemented in Gaussian 09 (23-31). Seven different possible conformer structures 2f1-2f7 were found. Energy differences [kcal/mol] for all minima were calculated with and without vibrational zero point energies (VZPE).
Results and discussion
Figure 1 shows the synthesis of new 1-phenylhex-5-en-1-ylacetamides 2a-f from N-butenylamines 1a-f using acetic anhydride as both reagent and solvent, under reflux (140 ºC). Acetamides 2a-f were easily obtained with yields between 66-92%. Optimal reaction time was established by both TLC and GC in about 4-6 h. Main IR vibration bands of allyl C=C-H and acetamide C=O are listed in Table 1. IR N-H tension and flexion bands in compounds 1a-f clearly disappeared after acetyl protection.
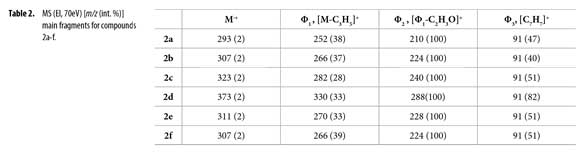
GC-MS (EI, 70eV) data are condensed in Table 2. Molecular ions corresponding to molecular mass of 2a-f appeared in all cases with low intensity; base peak Φ2 results after consecutive allyl [Φ1] and acetyl [(Φ1-C2H3O]+ fragmentation. Benzyl fragment Φ3 (m/z 91) is characteristic in all compounds.
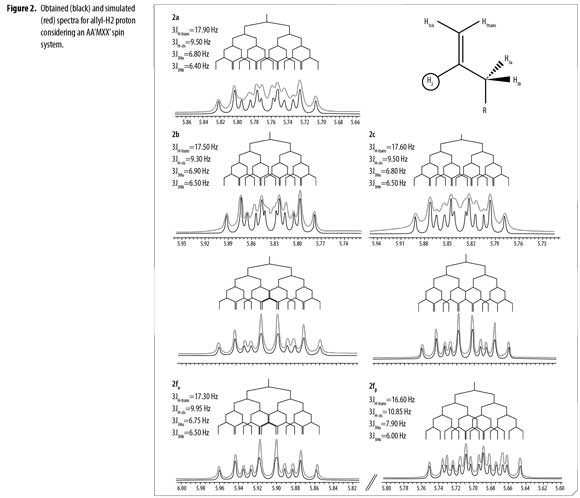
1H/13C and 2D NMR experiments confirmed the structures of compounds 2a-f. Due to a short relaxation time in 13C- APT NMR spectra for Co', a broad signal appeared; thus, in 2e it was not possible to assign the correct C o'-F coupling constant. Diastereotopic protons H3a and H3b appeared in 1H-NMR as a multiplet from 1.86 ppm to 2.59 ppm in all cases. Correct assignment of allyl-H2 coupling constants and multiplicity for compounds 2a-f was completed by comparative simulation considering a first order spectra (Δν/J >8) of an AA'MXX' spin system (32); spin simulation was carried out using Mestrenova v7.1.1 (test version) (33). Determined experimental values of chemical shifts and coupling constants range for the allyl system were used to simulate the H2 signal until an identical multiplet was obtained by direct comparison, as shown in Figure 2.
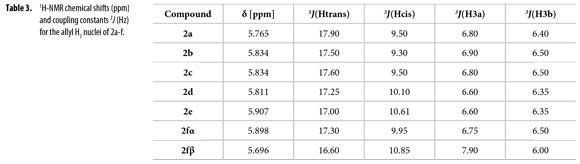
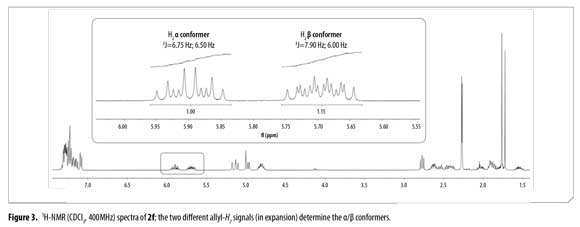
H2 proton chemical shifts and coupling constants for 2a-f allyl system are shown in Table 3. Compound 2f showed a single GC signal but a double group of signals in 1H and 13C-NMR; characteristic coupling constants for the allyl H2 proton were observed due to the existence of two different conformational isomers (α/β).
1H-NMR integral relation of conformers 2fαand 2fβ was 1:1.15 (Figure 3). 1H-NMR experiments showed that α and β conformational isomers ratio does not change in time at room temperature. Conformers 2fαand 2fβalso showed diastereotopic protons H3a and H3b as separated multiplets.
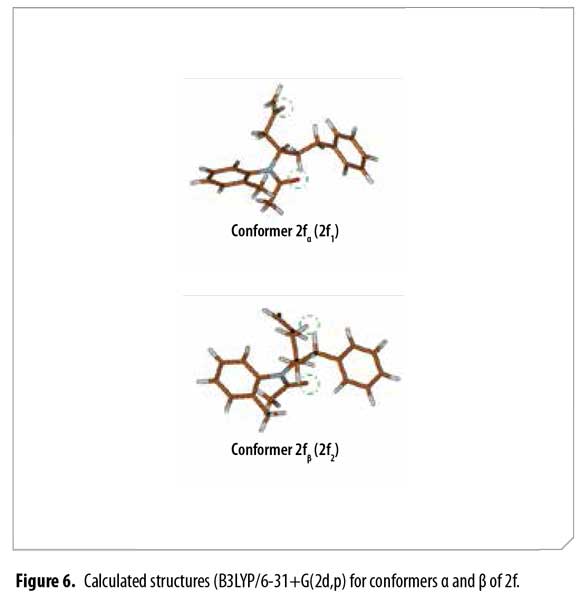
After conformational analysis of 2f, despite of including both, the electronic energy and the VZPE differences, the optimized structures 2f1-2f7 showed similar ΔE values, avoiding a direct assignment to explain the duplicity of the signals in 1H-NMR. Molden was used for displaying the molecular structures (34). These structures are qualitatively similar, differing mainly in the allyl group dihedral angles and slightly in the dihedral angles at other sigma bonds. Figure 4
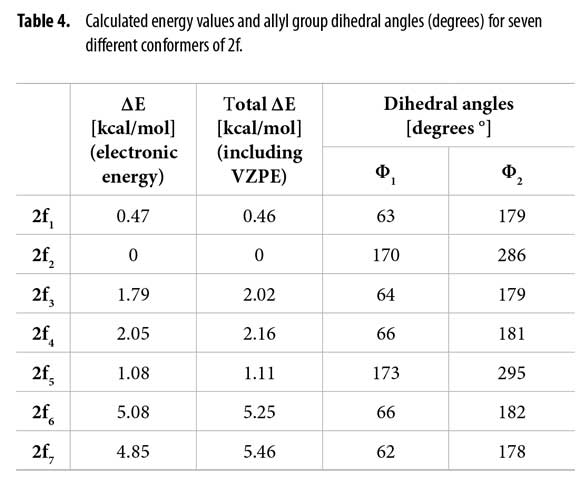
Table 4 shows the energies and the allyl group dihedral angles for each 2f conformer after optimization, taking 2f2 [lowest electronic energy at the B3LYP(6-31+G(2d,p)) level of theory] as reference for energetic differences.
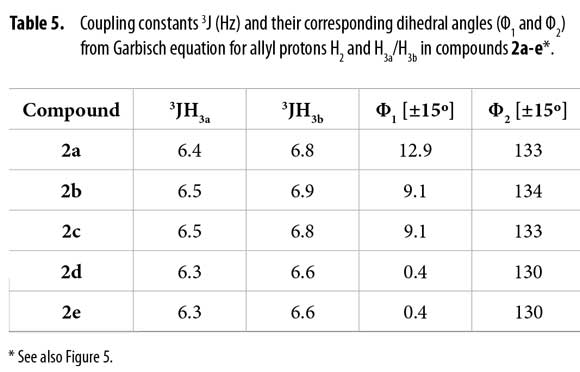
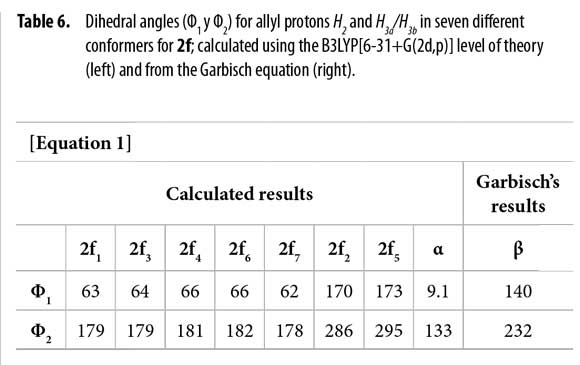
To correlate the H2 allyl 1H-NMR signals to a particular conformer, the proposed equation by Garbisch for allyl compounds [Equation 1] was used (35). The Garbisch equation is a modified Karplus equation involving also contributions of σ and π bonds. Some authors suggest that some modifications can be done to make this equation more precise (36-38). With the Garbish equation a relation between observed coupling constants (J) and the dihedral angle (Φ) for the allylic proton H2 and H3 for each conformer was determined, as shown in Tables 5 and 6.
Dihedral angles for conformers 2f1-7 found in our calculations and conformers 2fα and 2fβ obtained from the Garbisch equation are not equivalent, but approximated (Table 6) considering ±15° of uncertainty (32).
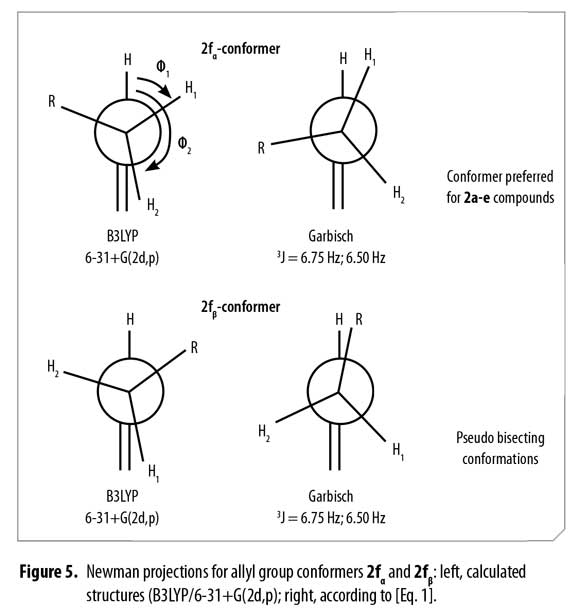
A comparison for both models (Figure 5) shows high similarity when a Newman representation of the allyl H2 and vicinal methylenic protons H3a and H3b are depicted. Considering this fact, it was possible to associate these two different positions for H3a and H3b protons to conformers α and β according to the observed 1H and 13C-NMR spectra, concluding that bulky groups (R and C=C) are located in a pseudo bisecting conformation in Newman's projections (Figure 5).
Thus, possible structures of α/β conformers were established according to their calculated energy, in each case. Allyl group disposition according to Garbisch's equation was within the approximation limits with calculated conformers 2f1 and 2f2 (Figure 6 ).
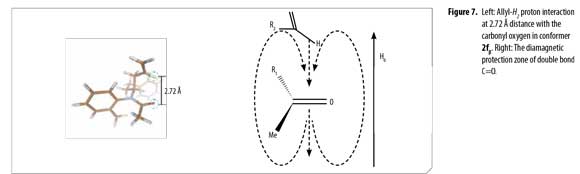
Analogous calculated structures for 2f differ basically on the spatial distribution of the allyl group and its dihedral angle; a 2.72 Å dipolar interaction between the allyl proton and the acetyl oxygen atom was observed in conformer 2fβ (Figure 7), explaining the chemical shifting to high fields in the 1H-NMR spectrum due to C=O anisotropic protection. Each conformer of 2f (α and β) was totally elucidated using 2D-NMR experiments (COSY, HSQC and HMBC).
Conclusions
An easy methodology to access new N-phenyl-N-(1-phenylhex-5-en-1-yl) acetamides 2a-f using acetic anhydride as reagent and solvent, including green chemistry principles was used. Using quantum chemical calculations and Garbisch's approximation it was possible to determine that compounds 2a-e prefer a single conformation similar to conformer 2fα. Existence of conformers 2fα and 2fβ explain the double signals observed in both 1H and 13C-NMR spectra for compound 2f. Coupling constants and chemical shifts values for H2 allyl proton signals of compounds 2a-f were described for each conformer and can be used as a comparative base in 1H-NMR allyl-H2 signal coupling constants assignation.
Acknowledgments
Authors express their acknowledgment to Universidad Industrial de Santander-UIS DIEF (internal project 5171) for financial support.
References
1. Schreiber, S. L. Target-Oriented and Diversity-Oriented Organic Synthesis in Drug Discovery. Science. 2000. 287: 1964-1969. [ Links ]
2. Goel, A. ; Kumar, V. ; Singh, S. P. ; Sharma, A. ; Prakash, S. ; Singh, C. ; Anand, R. S. Non-aggregating Solvatochromic Bipolar Benzo[f]quinolines and Benzo[a]acridines for Organic Electronics. J. Mater. Chem. 2012. 22: 14880-14888. [ Links ]
3. Lee, H. J. ; Xin, H. ; Park, S. M. ; Park, S. I. ; Ahn, T. ; Park, D. K. ; Jenekhe, S. A. ; Kwon, T. W. Synthesis and Properties of Diarylamino-Substituted Linear and Dendritic Oligoquinolines for Organic Light-Emitting Diodes. Bull. Korean Chem. Soc. 2012. 33: 1627-1637. [ Links ]
4. Vargas, L. Y. ; Castelli, M. V. ; Kouznetsov, V. V. ; Urbina, J. M. ; López, S.N. ; Sortino, M. ; Enriz, R. D. ; Ribas, J. C. ; Zacchino, S. In vitro Antifungal Activity of New Series of Homoallylamines and Related Compounds with Inhibitory Properties of the Synthesis of Fungal Cell Wall Polymers. Bioorg. Med. Chem. 2003. 11: 1531-1550. [ Links ]
5. Gómez-Barrio, A. ; Montero-Pereira, D. ; Nogal-Ruiz, J. J. ; Escario, J. A. ; Muelas-Serrano, S. ; Kouznetsov, V. V. ; Vargas-Méndez, L. ; Urbina-Gonzáles, J. M. ; Ochoa, C. AntiparasiticProperties of Homoallylamines and Related Compounds. Acta Parasitol. 2006. 51: 73-78. [ Links ]
6. Jacquemond-Collet, I. ; Hannedouche, S. ; Fabre, N. ; Fourasté, I. ; Moulis, C. Two Tetrahydroquinoline Alkaloids from Galipea officinalis. Phytochemistry. 1999. 51: 1167-1169. [ Links ]
7. Sridharan, V. ; Suryavanshi, P. A. ; Menéndez, J. C. Advances in the Chemistry of Tetrahydroquinolines. Chem. Rev. 2011. 111: 7157-7259. [ Links ]
8. Zanatta, F. ; Gandolfi, R. B. ; Lemos, M. ; Ticona, J. C. ; Gimenez, A. ; Clasen, B. K. ; Filho, V. C. ; de Andrade, S. F. Gastroprotective Activity of Alkaloid Extract and 2-phenylquinoline Obtained from the Bark of Galipea longiflora Krause (Rutaceae). Chem. -Biol. Interact. 2009. 180: 312-317. [ Links ]
9. Fournet, A. ; Barrios, A. A. ; Muñoz. V. ; Hocquemiller, R. ; Roblot, F. ; Cavé, A. ; Richomme, P. ; Bruneton, J. Antiprotozoal Activity of Quinoline Alkaloids Isolated from Galipea longiflora, a Bolivian Plant Used as a Treatment for Cutaneus Leishmaniasis. Phytother. Res. 1994. 8: 174-178. [ Links ]
10. Fakhfakh, M. A. ; Fournet, A. ; Prina, E. ; Mouscadet, J. F. ; Franck, F. ; Hocquemiller, R. ; Figadère, B. Synthesis and Biological Evaluation of Substituted Quinolines: Potential Treatment of Protozoal and Retroviral Co-infections. Bioorg. Med. Chem. 2003. 11: 5013-5023. [ Links ]
11. Kishore, N. ; Mishra, B. B. ; Tripathi, V. ; Tiwari, V. K. Alkaloids as Potential anti-tubercular Agents. Fitoterapia. 2009. 80: 149-163. [ Links ]
12. Ghassamipour, S. ; Sardarian, A. R. Friedländer Synthesis of Poly-substituted Quinolines in the Presence of Dodecylphosphonic Acid (DPA) as a Highly Efficient, Recyclable and Novel Catalyst in Aqueous Media and Solvent-free Conditions. Tetrahedron Lett. 2009. 50: 514-519. [ Links ]
13. Jacquemond-Collet, I. ; Benoit-Vical, F. ; Valentin, M. A. ; Stanislas, E. ; Mallié, M. ; Fourasté, I. Antiplasmodial and Cytotoxic Activity of Galipinine and other Tetrahydroquinolines from Galipea officinalis. Planta Med. 2002. 68: 68-69. [ Links ]
14. Crane, D. ; Holmes, R. S. ; Masters, C. J. On the Relative Rates of Synthesis and Degradation of Catalase in Vertebrate Tissues. Int. J. Biochem. 1978. 9: 589. [ Links ]
15. Incefy, G. S. ; Kappas, A. Inhibitory Effect of α-amanitin on the Induction of δ-aminolevulinate Synthetase in Chick Embryo Liver. FEBS Lett. 1971. 15: 153. [ Links ]
16. Zoumpoulakis, P. ; Camoutsis, Ch. ; Pairas, G. ; Sokovic, M. ; Glamoclija, J. ; Potamitis, C. ; Pitsas, A. Synthesis of Novel Sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as Potential Antibacterial and Antifungal Agents. Biological Evaluation and Conformational Analysis Studies. Bioorg. Med. Chem. 2012. 20:1569-1583. [ Links ]
17. Csütörtöki, R. ; Szatmári, I. ; Koch, A. ; Heydenreich, M. ; Kleinpeter, E. ; Fülöp, F. Syntheses and Conformational Analyses of new Napht[1,2-e][1,3]oxazino[3,2-c]quinazolin-13-ones. Tetrahedron. 2012. 68: 4600-4608. [ Links ]
18. Schulz, S. ; Bauer, T. ; Hoffbauer, W. ; auf der Günne, J. S. ; Doerr, M. ; Marian, C. M. ; Assenmacher, W. Stepwise Conversion of a single source precursor into Crystalline AIN by Transamination Reaction. J. Solid State Chem. 2008. 181: 530-538. [ Links ]
19. Fiebig, L. : Kuttner, J. ; Hilt, G. ; Schwarzer, M. C. ; Frenking, G. ; Schmalz, H-G. ; Schäfer, M. 2013. Cobalt Catalysis in the Gas Phase: Experimental Characterization of Cobalt (I) Complexes as intermediates in Regioselective Diels-Alder Reactions. J. Org. Chem. 2013. 78:10485-10493. [ Links ]
20. Mattheis, C. ; Wang, H. ;. Schwarzer, M. C. ; Frenking, G. ; Agarwal, S. Exploring Suitable Oligoamines for Phantom Ring-closing Condensation Polymerization with Guanidine Hydrochloride. 2013. Polym. Chem. 4: 707-716. [ Links ]
21. Tantillo, D. J. Walking in the Woods with Quantum Chemistry – Applications of Quantum Chemical Calculations in Natural Products Research. Nat. Prod. Rep. 2013. 30: 1079-1086. [ Links ]
22. Stewart, J. J. P. Optimization of Parameters for Semiempirical Methods. I. Method. J. Comp. Chem. , 1989. 10: 221-264. [ Links ]
23. Frisch, M. J. , et al. Gaussian 09, Revision A.1. , Gaussian Inc. , Wallingford CT, 2009. [ Links ]
24. Becke, A. D. Density‐functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993. 98: 5648-5652. [ Links ]
25. Lee, C. ; Yang, W. ; Parr, R. G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B. 1988. 37: 785-789. [ Links ]
26. Vosko, S. H. ; Wilk, L. ; Nusair, M. Accurate Spin-dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980. 58: 1200-1211. [ Links ]
27. Stephens, P. J. ; Devlin, F.J. ; Chabalowski, C.F. ; Frisch, M. J. Ab initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994. 98:11623-11627. [ Links ]
28. Ditchfield, R. ; Hehre, W. J. ; Pople, J. A. Self-consistent Molecular Orbital Methods. 9. Extended gaussian-type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971. 54: 724-729. [ Links ]
29. Hehre, W. J. ; Ditchfield, R. ; Pople, J. A. Self-consistent Molecular Orbital Methods. 12. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular-Orbital Studies of Organic-Molecules. J. Chem. Phys. 1972. 56: 2257-2262. [ Links ]
30. Hariharan, P. C. ; Pople, J. A. Influence of Polarization Functions on Molecular-Orbital Hydrogenation Energies. Theor. Chem. Acc. 1973. 28: 213-222. [ Links ]
31. Petersson, G. A. ; Al-laham, M. A. A Complete Basis Set Model Chemistry. II. Open-Shell Systems and the Total Energies of the First-Row Atoms. J. Chem. Phys. 1991. 94: 6081-6090. [ Links ]
32. Hoye, T. R. ; Hanson, P. R. ; Vyvyan, J. R. A Practical Guide to First-Order Multiplet Analysis in 1H-NMR Spectroscopy. J. Org. Chem. 1994. 59: 4096-4103. [ Links ]
33. Mestrenova, versión 7.1.1; MestreLabresearch S.L. : Santiago de Compostela, España, 2011. http://www.mestrelab.com [Access March-2012] [ Links ].
34. Schaftenaar, G. ; Noordik, J. H. Molden: A Pre- and Post-processing Program for Molecular and Electronic Structures. J. Comput. -Aided Mol. Des. 2000. 14: 123-134. [ Links ]
35. Garbisch, E. W. Conformations VI. Vinyl-Allylic Proton Spin Couplings. J. Am. Chem. Soc. 1964. 86: 5561-5564. [ Links ]
36. Karplus, M. Vicinal Proton coupling in Nuclear Magnetic Resonance. J. Am. Chem. Soc. 1963. 85: 2870-2871. [ Links ]
37. Bally, T. ; Rablen, P. R. Quantum-Chemical Simulation of 1H-NMR Spectra. 2. Comparison of DFT-Based Procedures for Computing Proton-Proton Coupling Constants in Organic Molecules. J. Org. Chem. 2011. 76: 4818-4830. [ Links ]
38. Vyas, D. M. ; Hay, G. W. Studies on the Synthesis of Novel Carbohydrates with Sulphur in the Ring. Part II. Analogues of Derivatives of Unsaturated Deoxy-ulo-pyranosidonic Acids via Diels-Alder Reactions with Methyl Cyanodithioformate. J. Chem. Soc. Perkin Trans. I. 1975. 2: 180-186. [ Links ]