INTRODUCTION
Milk and dairy products are daily consumption foodstuffs, considered as important sources of energy, and of a variety of bioactive substances positively associated with human health, such as proteins and peptides, oligosaccharides, lipids, minerals and vitamins 1. Milk fat is mainly composed of triacylglycerols (~98%), minor amounts of mono and diacylglycerols (~2%), phospholipids (~1%), sterols (~0.5%), free fatty acids (0.1%) and traces of fat-soluble vitamins 2. The high concentration of saturated fatty acids (mainly that of palmitic, myristic and lauric acids) in the milk's lipid fraction has generated some concern, because of their negative effects on human health, especially related to the increased risk of acquiring cardiovascular diseases 3. However, milk's lipid fraction also contains mono and polyunsaturated fatty acids, such as oleic acid (C18:1 cis9), and fatty acids belonging to the omega-3 (C18:3) and omega-6 families (C18:2), which are considered beneficial to human health, especially for reducing the level of triglycerides and LDL cholesterol, and for enhancing the levels of HDL cholesterol 4)(5. Since the concentration of these fatty acids is relatively low for providing health benefits, significant efforts have been devoted to increasing their content in milk and dairy products, in order to produce foodstuffs with improved nutritional value. The main employed strategies are based on the manipulation of the feed and dietary regimen of the animals, and on the modification of the technological processes used in the manufacture of dairy products 6)(7)(8.
Conjugated linoleic acid is a generic term used for describing the geometrical and positional isomers of linoleic acid (C18:2 cis9, cis12) with a conjugated double bond system 9. Milk fat is the richest natural source of CLA, with concentrations typically ranging between 2 and 37 mg/g fat 10. The Table 1 presents the predominant distribution of the CLA-isomers in milk and dairy products.
Table 1 Distribution of the main CLA-isomers in milk and dairy products (Adapted from Bauman and Lock (11)).
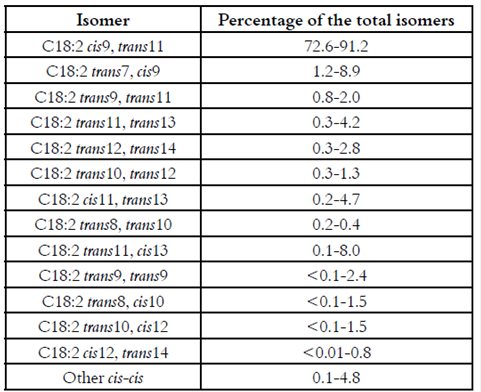
As it can been appreciated in Table 1, the cis-9, trans-11-octadecadienoic acid (C18:2 cis9, trans11), also known as rumenic acid, is the predominant isomer, representing between 75 and 90% of the total CLA-isomers (12). The second most abundant isomer is the C18:2 trans7, cis9, represents about 10% of the total CLA-isomers. The remaining isomers, including the trans-10, cis-12-octadecadienoic acid (C18:2 trans10, cis12), are present in small concentrations, mostly around 0.5% (11).
Among the identified CLA-isomers, the C18:2 cis9, trans11 and the C18:2 trans10, cis12, are considered as molecules biologically active, due to their protective effects against various common diseases such as obesity 13)(14, atherosclerosis 15)(16, diabetes 17)(18, some chronic inflammatory diseases 1 and cancer 4)(19)(20. Anticarcinogenic effects have been observed in different cancer types, with doses varying between 55 mg and 3.5 g CLA/day 21)(22)(23, but the most important results have been reported regarding breast cancer 11.
Numerous studies have evaluated the factors affecting the content of CLA in milk and dairy products, because these foodstuffs are the main source of CLA in the human diet, providing about 70% of the total CLA daily intake, which varies between 70 and 430 mg/day 11)(24. However, including the conversion of vaccenic acid in the human body by the D9-desaturase enzyme, an average intake of 650 mg/day can be achieved 24. Since these intake values are lower than those recommended for achieving beneficial effects on human health (3-4 g CLA/day), various technological alternatives have been investigated in order to increase the CLA concentration in milk and dairy products, and hence the daily CLA intake of the population. The main investigated alternatives comprise the manipulation of the feed and dietary regimen of the animals, and the milk fermentation with CLA-producing bacteria (bacteria possessing linoleate isomerase activity), alone or in combination with specific starter cultures 24.
Taking into account that the development of dairy products with high concentrations of CLA might represent an interesting economic opportunity, due to important participation of the dairy industry in the functional foods market, the objective of this work is to present a comprehensive review of: (i) The content of CLA in milk and the main factors affecting its concentration; (ii) the influence of the technological treatments normally applied to milk on the concentration of CLA; and (iii) the effects of the milk fermentation on the CLA concentration of fermented dairy products, and the main challenges of this technological process, considered as promissory for obtaining dairy products naturally enriched with CLA. To the best of our knowledge, articles combining these topics have not been yet published.
METHODS
The data presented in this review were collected from scientific publications and thesis published between 2000 and 2014. The literature search was achieved using the following keywords: conjugated linoleic acid, milk, fermented milks and yogurt. From a total of about 300 documents found, 103 were selected and analyzed on the basis of their pertinence, relevance and technical-scientific quality. Taking into account that the concentration of CLA was not always expressed in the same units in the published papers, in some cases conversion factors were used, for making the comparisons between the data of the selected references.
CLAIN MILK: SYNTHESIS, VARIABILITY AND EFFECTS OF THE TECHNOLOGICAL PROCESSES CLA synthesis
The formation of CLA-isomers in milk is carried out by means of isomerization and biohydrogenation reactions of the unsaturated fatty acids presented in the feed and dietary regimen of the animals. These reactions are produced by the rumen bacteria and the enzymatic activity of the Δ9-desaturase in the mammary gland 10. The linoleic acid ingested in the diet is firstly isomerized to rumenic acid (C18:2 cis9, trans11) by the cis-12, trans-11 isomerase enzyme, and then biohydrogenated to vaccenic acid (C18:1 trans11) in the rumen by the bacteria Butyrivibrio fibrisolvens10. This anaerobic bacteria is also responsible of the formation of the rumenic and vaccenic acids from the α-linolenic (C18:3 cis9, cis12, cis15) and ɣ-linolenic (C18:3 cis6, cis9, cis12) acids 25. If the initial isomerization of the C18:2 cis9, cis12 acid involves the cis12 double bond, the isomer C18:2 cis9, trans11 is produced, whereas if the initial double bond isomerized is the cis9, the isomer C18:2 trans10, cis12 is mainly produced 11.
In the mammary gland, the biochemical reaction of the Δ9-desaturase enzyme with the vaccenic acid leads to the production of 70-90% of the C18:2 cis9, trans11 isomer 26. The other CLA-isomers, including the C18:2 trans10, cis12, are intermediate products of the biohydrogenation reactions in the rumen. Thus, their concentration in milk are lower than those of the rumenic acid 27. Detailed studies on the lipid metabolism in the rumen and its effects on the CLA-isomers production have been recently published 10)(28.
Variability
The concentration of CLA in cows' milk normally ranges between 2 and 37 mg/g fat 10. Recent studies have reported CLA concentrations of about 13.5 mg/g fat, in milks from “Sabana de Bogotá” 29. However, pasteurized milks with high content of CLA (30 mg/g fat) can be found in markets of various countries such as Canada and Spain 30.
The lactation stage, the rumen microflora and the type of feed offered to the animals are some of factors influencing the concentration of CLA in milk 31)(32. However, taking into account that the CLAisomers are mainly synthesized from the unsaturated fatty acids ingested by the animals, the diet is the factor affecting the most the content of CLA in milk fat 10. According to Bell and Kennelly 33, the concentration of CLA in milk can be increased up to 10 fold, by the manipulation of the animal's diet. Various studies have demonstrated that the CLA content in milk increases significantly by using feed systems based on the consumption of fresh pasture, whose content of polyunsaturated fatty acids is high 32)(34. On the contrary, diets based on the supply of grains and preserved forage, lead to a reduction in the CLA concentration in milk, due to the pH decreases and changes in the microbial composition of the rumen 35. The fodder type and the regrowth age would also have an effect on the concentration of CLA isomers in milk 35. Other researches have shown that the addition of supplements rich in linoleic acid, such as soybean, olive, canola, sunflower and fish oils, can slightly increase both the CLA content and the concentration of unsaturated fatty acids in milk and dairy products 3)(9)(36. Nevertheless, is important to note that the use non-protected oils, such as vegetable and fish oils, generally produces milk fat depression and significant decreases in the milk yield, as well as increases in some trans fatty acids, such as C18:1 trans11, C18:1 trans10 y C18:2 trans9, cis11 37. The complete details of the main diet effects on the concentration of CLA isomers in milk have been the subject of recent review articles 10)(38)(39)(40.
Effects of the technological processes
The studies of the influence of the technological processes normally applied to milk for manufacturing dairy products on the CLA concentration are very diverse. The reported results are controversial due to their remarkable differences, suggesting that the CLA concentration in dairy products mainly depends on the CLA content in the raw milk, which, as mentioned above, is principally influenced by the diet regimen of the animals, and in a less manner by the reactions produced during technological processes used in milk processing.
Thermal processing
Milk is thermally processed in order to eliminate pathogens and make it safe for human consumption. The effects of the thermal treatments on the main milk components (lactose, proteins, fat and vitamins) have been extensively studied 41. However, specific studies related to the effect of the thermal processing of milk on the CLA concentration are scarce and their results present much variation.
According to some authors, the heating of milk fat in the presence of proteins in aqueous solution can generate an increase in the concentration of CLA 42. Other studies indicate that the microwave heating of milk and the UHT treatment (140ºC, 4 s), can produce significant decreases in the CLA content, due to oxidation reactions of the fat and the generation of hydroperoxides that could cause conversion or degradation of the CLA isomers 43)(44. Similar results were found after heating milks by different pasteurization treatments (HTST: 77.2ºC, 16 s; LTLT: 60ºC, 20 min; and ultra-pasteurization: 138ºC, 3 s) 45)(46)(47. On the contrary, it has been also reported that various pasteurization treatments (85ºC, 16 s; 95ºC, 5 min; 63ºC, 30 min; 70-90ºC, 5 min) and UHT processing do not generate significant changes on the CLA content of milk 9)(43)(48. Recent investigations evaluated the influence of various thermal treatments (pasteurization (72ºC, 30 s); HTST (85ºC, 30 s); UHT (135ºC, 30 s); UHT (150ºC, 5 min); sterilization (121ºC, 15 min) and microwave heating (650 W, 1.30 min)) on the milk' CLA concentration 49. The HTST pasteurization produced a sigmatropic rearrangement of the CLA-isomers, increasing the concentration of the C18: 2 cis9, trans11 isomer. The sterilization treatment generated the isomerization of the linoleic acid into the C18:2 trans9, trans11 isomer, while the content of the C18:2 cis9, trans11 isomer in the milk heated by microwaves was significantly higher than that of the untreated milk. In addition, the concentration of CLA in milks treated by means of the UHT process gradually decreased during the storage period 50. These results were attributed to the presence of oxygen during the HTST pasteurization and the microwave treatment, allowing the formation of protein radicals, which can react with the linoleic acid, thus increasing the concentration of the C18:2 cis9, trans11 isomer 49. These results are in agreement with previous findings 51, which indicate that the thermal processing of milk may alter the CLAisomers' distribution, but their total concentration remains constant.
The retention kinetics of CLA and trans-vaccenic acid of milks thermally treated within the temperature interval between 90 and 120°C, was recently investigated 23. Both lipids were oxidized in great extent when the temperature increased from 90 to 120°C. After 60 minutes of heating at 90°C, the retention percentage of CLA varied between 68 and 71%, while in the milks treated at 120°C during 15 minutes, the retention percentage was only 15-21%.
Although there is no a rule concerning the effects of thermal processing on the CLA concentration in milk, the normal thermal processes applied to milk could affect not only the concentration of CLA in milk, but also the CLA-isomer distribution. More research is needed in order to establish the mechanisms of these possible changes.
High pressure processing
The potential of emerging technologies such as high hydrostatic pressure has been widely studied as non-thermal alternative to milk pasteurization. However, few studies have evaluated the influence of this technology on the concentration of CLA in milk and dairy products. The fatty acid composition (including that of CLA-isomers) of milks homogenized at high pressures (up to 350 MPa), did not present significant variations in comparison to the control samples 52. Milks naturally enriched with CLA, did not show significant changes in the fatty acid composition, including the CLA-isomers, after processing at high pressures (400 MPa, 15 min, 25°C) 49. Recent reports indicate that the combined effect of high pressure with temperature may produce an increase in the retention of CLA 30)(53. The retention kinetics of the CLA-isomers of milks treated by high pressure sterilization (100-600 MPa, 90-120°C) was well described by the Weibull model 6. The retention of CLA decreased with an increase of temperature, but more CLA was retained with an increase of pressure. In addition, the processing conditions at which commercial sterilization may be achieved (120°C and 600 MPa with 3 min of holding time) allowed to retain more than 80% of the CLAisomers. This high retention value was attributed to isomerization reactions, instead oxidation reactions, taking into account that in the presence of oxygen the CLA isomerization is thermally induced through a free radical mechanism in which oxygen is consumed during the reaction 51. However, the retention of CLA decreased progressively during storage, being approximately 36% after 60 days at 25°C 30.
Refrigeration
Some studies have evaluated the influence of the refrigeration process of milk and dairy products on the content of CLA-isomers. In general, the results indicate that refrigerated storage does not significantly affect the concentration of CLA in milk and dairy products 54)(55. However, there are reports that indicate important decreases of the C18:2 cis9, trans11 and C18:2 cis10, trans12 isomers of skim milk enriched with CLA after three weeks of refrigerated storage, possibly due to the activity of microbial lipases 45. Leite, Lima and Baptista 46 found a 12% reduction of the C18:2 cis9, trans11 isomer in commercial UHT milk after two months of refrigerated storage at 6-7ºC. Similarly, significant decreases of the C18:2 cis9, trans11; C18:2 cis11, trans13; C18:2 cis10, cis12 and C18:2 cis10, trans12 isomers were found during refrigerated storage of fresh cheese, as a consequence of an excessive microbial growth 54.
Fermentation
The ability of microorganisms for producing CLA from linoleic acid depends on the activity of their linoleate isomerase, enzyme catalyzing this reaction 24. Species expressing linoleate isomerase activity can be divided in two groups: bacteria that produce mainly C18:2 cis9, trans11 from linoleic acid, and bacteria able to produce mainly C18:2 trans10, cis12 from linoleic acid. In addition to the rumen bacteria, this enzyme has been found in strains of Bifidobacterium, Enterococcus, Lactobacillus, Lactococcus, Propionibacterium and Streptococcus, which have been classified as potential CLA-producers 56)(57)(58. Genetic sequences of the linoleate isomerase enzymes from strains of B. dentium, B. breve, Lc. lactis ssp. lactis, L. acidophilus, L. plantarum, L. reuteri, P. acnes, and Rhodococcus erythropolis are available in GenBank 24.
The influence of the processing conditions for producing of CLA in vitro using microorganisms has been largely studied 24)(58)(59)(60. The concentration of linoleic acid, pH, temperature and the microbial growth stage are the most important factors on the bioproduction yield of CLA 24. Although most of the investigated microorganisms are normally used as starter cultures or as probiotics in the manufacture processes of fermented milks, is important to note that the obtained results of in vitro essays in MRS medium, may not be extrapolated to the milk fermentation processes, because of the changes and multiple interactions characterizing the milk-based substrates. Some recent findings are discussed below.
Studies have shown that strains of B. breve could be used as starter cultures for the development of functional milk products with high concentrations of bioactive lipids such as CLA 61)(62)(63)(64)(65)(66. Conversions of linoleic acid into CLA up to 74% were obtained using three strains of B. breve (ZL12-28, 29M2 and M7-70) isolated from breast milk 62. These strains cultured in MRS mediun and reconstituted skimmed milk, produced different CLA-isomers (C18:2 cis 9, trans11; C18:2 trans10, cis 12 y C18:2 trans 9, trans 11) in amounts varying between 160- 170 mg CLA/mL. Coakley et al. 64 obtained CLA (mainly the C18:2 cis9, trans11 isomer) by using strains of B. breve and B. dentium. Among 36 strains of Bifidobacterium, B. breve, B. bifidum, and B. pseudolongum ssp. pseudolongum converted 20 to 54% of linoleic acid into CLA (C18:2 cis9, trans11 y C18:2 trans9, trans11) in MRS medium 63. Similarly, strains of B. breve were able to produce CLA (mainly the C18:2 cis9, trans11 isomer) in skimmed milk supplemented with linoleic acid (0.5 mg/mL), with conversions up to 23% 61. The conversion was higher (39%) when strains of B. bifidum were cultured in buffalo milk supplemented with linoleic acid at low concentrations (0.2 mg/mL) 67. B. breve LMG 13194 and B. pseudolongum ssp. pseudolongum LMG 11595 produced higher conversion of linoleic acid into CLA in MRS medium, in comparison to B. breve LMG 11084, B. breve LMG 11040, and B. breve LMG 11613, confirming that the CLA production is strain-dependent, probably because of the differences in the genetic sequences of the linoleate isomerase enzyme of each microorganism 68.
Numerous strains of Lactobacillus have shown high linoleate isomerase activity and can biosynthesize CLA from linoleic acid 69)(76. L. plantarum ZS2058 converted more than 50% of linoleic acid into CLA (C18:2 cis9, trans11 y C18:2 trans9, trans11) in MRS medium, while strains of L. bulgaricus, L. crispatus, L. gasseri and L. helveticus converted only 10- 20% of linoleic acid in three CLA-isomers (C18:2 cis9, trans11; C18:2 trans9, trans11 and C18:2 trans10, cis12) 69. Recently, high conversions (86.4%) of linoleic acid into CLA using permeabilized L. acidophilus cells were reported 77. The whole cells converted only 38.5%. The permeabilized cells were recycled ten times without showing a significant decrease in their catalytic activity. Similar results were obtained by Lee et al. 78, who reported 35% conversion of linoleic acid into CLA by using immobilized L. reuteri cells. The cells were recycled five times without presenting negative effects on the production of CLA. Lin et al. 70 evaluated the CLA production capacity of strains of L. acidophilus, L. delbrueckii ssp. bulgaricus and L. delbrueckii ssp. lactis, in MRS medium enriched with 12% skimmed milk and linoleic acid in concentrations ranging between 0 and 5000 μg/mL. An increase in the concentration of CLA was observed in the substrates added with linoleic acid. L. acidophilus presented the highest CLA production capacity (23.0-105.5 μg/mL), when incubated during 24 hours at 37ºC, with a linoleic acid addition of 1000 μg/mL. Further increases in the concentration of linoleic acid (from 1000 to 5000 μg/mL), as well as in the incubation time (from 24 to 48 hours), did not conduce to significant increases in CLA production. Similarly, strains of L. acidophilus, L. plantarum, L. casei, L. delbrueckii ssp. bulgaricus, and Streptococcus thermophilus produced CLA in MRS medium enriched with linoleic acid at 0.50 mg/mL 75.
The CLA production capacity of more than 250 strains from the genera of Lactobacillus, Streptococcus, Pediococcus, Leuconostoc, Propionibacterium, Bifidobacterium, Weissella, Aquaspirillum, Enterococcus, Tetragenococcus, Aerococcus, Butyrivibrio and Lactococcus was studied by Kishino et al. 79. Strains from the genera of Enterococcus, Pediococcus, Propionibacterium, and Lactobacillus produced considerable amounts of CLA in MRS medium supplemented with linoleic acid. Two strains of P. freudenreichii ssp. freudenreichii and one strains of P. freudenreichii ssp. shermanii converted 60 to 90% of linoleic acid into CLA (C18:2 cis9, trans11) in MRS medium 80, while strains of Lc. lactis ssp. lactis biovar diacetylactis and Leuconostoc mesenteroides ssp. mesenteroides were able to produce CLA (0.20 mg/mL) in skimmed milk added with hydrolyzed sesame oil 81
CLA IN FERMENTED MILKS
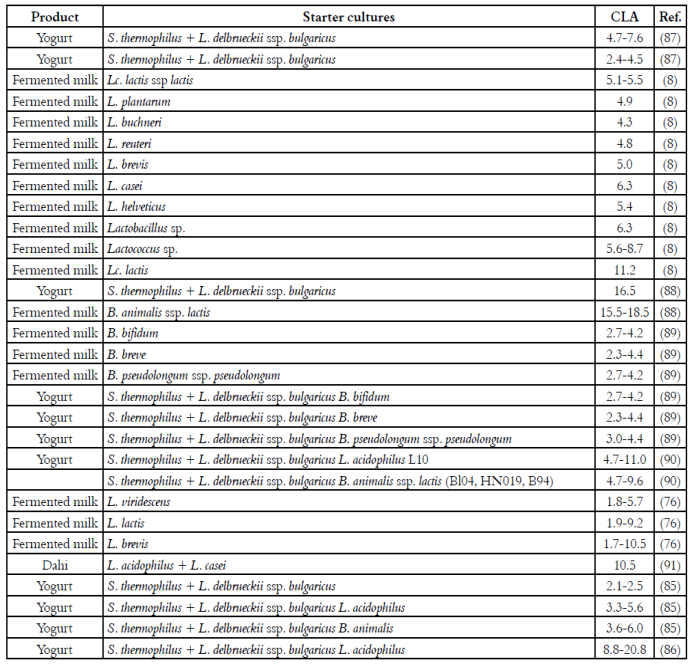
Fermented milks such as yogurt are considered healthy foods, due to their beneficial effects on human health. The concentration of CLA in fermented milks normally varies between 3.4 and 8.8 mg/g fat 24)(82. Recent reports indicate that the content of CLA in commercial yogurt and kumis from Colombia ranges between 4.5 and 8.2 and between 7.6 y 22.6 mg/g fat, respectively 82)(83. Table 2 presents the concentration of CLA in various fermented milks, as well as the starter cultures employed in the fermentation process.
Various studies have evaluated the influence of the milk fermentation on the concentration of CLA. The reported results are controversial. While some authors report that fermentation of milk does not affect the CLA content 84)(85)(86, others suggest that the use of lactic acid bacteria may increase 8)(55)(57 or even reduce 47 the concentration of CLA in fermented milk products. More research is needed in order to elucidate if the fermentation of milk can be used as an alternative method for manufacturing dairy products with high CLA content. However, from the published results it can be summarized that:
(i) The nature of milk can influence either the amount or the type of CLA-isomers in fermented milks. Studies have shown that the CLA content of yogurts from sheep milk is higher than that of yogurts from cow's milk (4.7-7.6 vs. 2.4-4.5 mg/g fat) 87, while the CLA concentrations of Greek yogurts elaborated with milks from cow, sheep and goat varied between 12.8-15.1, 4.1-12.5 and 4.3-9.8 mg CLA/g fat, respectively 92. After 14 days of refrigerated storage at 5°C, the concentration of CLA significantly increased in yogurts from goat milk, but it was significantly reduced in those from cow's milk 87. During the manufacturing of Dahi (fermented product from buffalo milk, similar to yogurt) Yadav et al. 91 found a significant increase (about twice) in the amount of CLA, when using L. acidophilus and L. casei as starter cultures. The refrigerated storage at 4°C did not affect the concentration of the CLA-isomers. On the other hand, the CLA content of organic fermented milks, produced using strains of B. animalis ssp. lactis in combination with S. thermophilus, was slightly higher than the CLA concentration of the starting milk 88)(93)(94. In contrast, there was no formation of CLA in fermented milks with strains of Bifidobacterium, despite having been chosen for their ability to produce CLA in vitro, and being cultured in substrates rich in linoleic acid 89.
(ii) The modification of the substrate can also affect the concentration of CLA in fermented milks. For example, the production of CLA was increased in milks fermented with Lc. lactis I-01 from substrates added with sunflower oil (0.1 mg/mL) 95. Also, the fermentation of milk enriched with linoleic acid (0.1%) with lactic cultures and L. acidophilus, showed a significant increase in the CLA content, without negative effects on the sensory properties 96. High values of CLA (5.58 and 7.06 mg/g fat) were obtained in low fat yogurts, in comparison to the reported in other studies (1.7 mg/g fat 97 and 5.25 mg/g fat, from milk added with 4.40 mg CLA/g fat 55). Slightly elevated concentrations of the C18:2 cis9, trans11 and C18:2 trans10, cis12 isomers were obtained in fermented milks elaborated from substrates added with hydrolyzed soybean oil, which were cultured with P. freudenreichii ssp. freudenreichii 23, P. freudenreichii ssp. shermanii 56, P. freudenreichii ssp. shermanii 51, L. rhamnosus and yogurt starter cultures (L. delbrueckii ssp. bulgaricus and S. thermophilus) 98. The combination of probiotics and yogurt starter cultures led to higher concentrations of CLA than those obtained without probiotic cultures. It has been also found that the addition of monolinolein favors the production of CLA in milks fermented with B. breve LMC 017 66. Moreover, the fatty acid profile of yogurts fermented with S. thermophilus and L. delbrueckii ssp. bulgaricus and strains of B. lactis Bl04 or B94 was enhanced by the addition of açai pulp to the starting milk, which provided an extra amount of unsaturated fatty acids, which may act as CLA precursors 99. On the contrary, the addition of oils rich in unsaturated fatty acids did not modify the CLA concentration of creams fermented with various probiotic cultures 100. Similar results were obtained by Xu et al.101, who did not observe significant increases in the concentration of CLA when milk added with 1% fat was cultured with L. rhamnosus and yogurt starter cultures (L. bulgaricus and S. thermophilus). The content of the C18:2 cis9, trans11 isomer remained constant during the refrigerated storage, while the concentration of the C18:2 trans10, cis12 isomer increased up to 3.3 fold after two weeks of storage. The addition of dietary fibers from apple, banana and passion fruit processing by-products led to a significant increase of the polyunsaturated and short chain fatty acids of yogurts fermented with four different strains of probiotic cultures: L. acidophilus L10 and B. animalis ssp. lactis BL04, HN019 and B94 90. A synergistic effect between the type of fiber and the probiotic strain on the CLA concentration was found. In yogurts without fiber addition, the content of the C18:2 cis9, trans11 isomer varied between 4.7 and 8.0 mg/g fat, while in the fiber-added yogurts, the values were slightly higher, varying between 5.4 and 11.2 mg/g fat. The control yogurt samples, co-fermented with B. animalis ssp. Lactis, showed CLA concentrations higher than those cofermented with L. acidophilus L10 (8.0 vs. 4.7 mg/g fat), suggesting again that the CLA products are strain-dependent. In spite of this, the highest increases in the CLA concentration were obtained in the samples added with fibers from banana and passion fruit, co-fermented with L. acidophilus L10. The fiber from passion fruit promoted the increase in the CLA content of all yogurt samples. These results are in agreement with previous studies in which the combination of strains of L. acidophilus and B. animalis, and prebiotics such as fructooligossacharides (FOS), favored the production of CLA during the manufacture of fermented milks (85, 102). Milks added with FOS (2%) and co-fermented with L. acidophilus and B. animalis presented concentrations of CLA almost three fold higher (2.7 and 2.9 fold, respectively), in comparison to the control yogurt samples without prebiotic addition 85. However, the addition of FOS did not cause significant variations in the yogurt samples without the addition of probiotic cultures, indicating that there is a synergistic effect, because the use of L. acidophilus and B. animalis led to a significant increase in the concentration of CLA, when comparing with the control samples fermented only with yogurt starters (3.3 vs. 2.1 and 3.6 vs. 2.1 mg/g fat, respectively). During refrigerated storage, the CLA concentration of the yogurts remained constant after 28 days. Moreover, the supplementation (4% w/w) with other prebiotic compounds such as maltodextrin, oligofructose and polydextrose was reported to generate significant increases in the concentration of CLA in milks fermented with S. thermophilus and L. acidophilus102. On the contrary, it has been reported that the addition of sucrose, fructose and lactose (60 g/L) led to an inhibition of the CLA production in yogurts elaborated using L. acidophilus and L. delbrueckii ssp. bulgaricus 103.
In one study evaluating the CLA production capacity of 155 different dairy starter cultures (Lactococcus, Lactobacillus, Streptococcus y Bifidobacterium), and 11 commercial yogurt starter cultures, it was found that 13 species from Lactococcus and seven from Lactobacillus, produced CLA in whole milk, in concentrations ranging between 4.3 and 11.2 mg/g fat 8. On the contrary, none of the commercial starter cultures produced CLA during the fermentation process.
CONCLUDING REMARKS
In this work a comprehensive review on the concentration of CLA in milk, and on the influence of different technological processes normally applied to milk on the content of these bioactive compounds has been presented. Special emphasis was done in fermented milks. Taking into account the biological importance of the CLA-isomers, an increase in their intake would be beneficial to the human health. Likewise, the development of dairy products with high concentrations of CLA could have a great impact on the market of functional foods.
Because milk and dairy products are the foodstuffs providing the highest amounts of CLA in the human diet, various technological alternatives have been evaluated for increasing the concentration of CLA in these products in a natural manner. The manipulation of the animals' diet has allowed increasing the content of CLA in milk, while the fermentation using microorganisms having linoleate isomerase activity has been employed as a promissory technological alternative for the manufacture of fermented milk products with high content of CLA. However, many factors can influence the concentration of CLA in milk and fermented milks. Although the mechanisms governing the biosynthesis of CLA during the milk fermentation are still unknown, it is evident that this reaction mainly depends on the type of strain. Various strains of food grade microorganisms such as Bifidobacterium, Lactobacillus and Propionibacterium have showed CLA production capacity from linoleic acid in milk-based culture mediums, but it is still unknown why some strains produce greater amounts than others.
The substrate composition is also an important factor in the production of CLA in fermented milks. The addition of fibers from fruits and prebiotics such as maltodextrine and FOS, has showed to be an effective method for enhancing the concentration of CLA in fermented milks manufactured using traditional yogurt starter cultures in combination with probiotic strains such as L. acidophilus, which could be an indicator of synergetic effects taking place in the production of CLA in this kind of dairy products. Likewise, the supplementation with linoleic acid has led to significant increases in the concentration of CLA in yogurt and other fermented milks. However, the addition of free fatty acids can on one hand increase the production costs, and on the other hand, be restricted because of the toxicity that this kind of compounds may provide at high concentrations.
The use of permeabilized cells may be a low cost technological alternative for the production of CLA, because it overcomes the problems related to the purification of the linoleate isomerase enzyme from microorganisms, and to the low permeability of the linoleic acid into the microbial cells. In addition, these biocatalysts can be reused several times without noticeable loss of activity.
Finally, although several studies have reported increases in the concentration of CLA in milk and fermented milk products, they are fairly moderate, and the obtained levels of CLA are significantly lower than those recommended to achieve therapeutic effects. More research is needed on the characterization of the linoleate isomerase enzymes produced by microorganisms, and on the appropriate process conditions (i.e. type of strains and substrate supplementation) to produce fermented dairy products with high concentrations of CLA..