Introduction
Functional oils are defined as oils that have an action beyond their nutritional value (Murakami et al., 2014) and are not derived from essences or spices (Bess et al., 2012), but from oil plants such as castor seeds (Ricinus communis) and cashew nutshell (Anacardium occidentale;Silva et al., 2014).
Castor seeds contain ricin, a water-soluble toxin. Although these seeds are toxic, castor oil is not, because ricin is not fat-soluble (Gaillard and Pepin, 1999). Additionally, ricinoleic acid has antimicrobial properties (Maenz and Forsyth, 2005). Antimicrobial action in castor oil comes from anacardic acid and cardol. Anacardic acid has been shown to inhibit the growth of gram-positive bacteria (Lima et al., 2000). On the other hand, oil from cashew nutshell has also antimicrobial and antioxidant activities (Oliveira et al., 2011). This study was conducted to determine how feeding functional oils could affect blood parameters and presence of Escherichia coli, Clostridium spp, and Salmonella spp in sheep feces.
Materials and methods
Ethical considerations
The study was approved by the Animal Ethics Committee of Instituto Federal Goiano de Rio Verde, Brazil (number 21/11, February 09, 2012).
Animals
The experiment was conducted with five male Santa Inés sheep. The sheep were 14 months old and weighed 35 Kg. Sheep were kept in confinement and fed diets formulated to fulfill NRC (2007) requirements during 14 d. Functional oils (FO) consisted of a mixture of cashew oil, castor oil, and silica. The oils included cardol (40 g/Kg), ricinoleic acid (90 g/Kg), and cardanol (200 g/Kg). Sheep were allocated in individual boxes for adaptation during two weeks before the experimental period. The boxes were cleaned daily and washed weekly. A 5×5 Latin square design was used, with five treatments: 190, 285, 380, 570, and 675 g/t FO in the diet. On the 15th d, blood samples were collected and analyzed for white cell count (WCC), total protein (TP), glucose, and blood urea nitrogen (BUN). The reference values for TP, glucose and BUN are 6.0-7.9 g/dL, 50- 80 mg/dL, and 17.12-42.8 mg/dL, respectively (Meyer and Harvey, 2004). Fecal samples were collected directly from the rectum to isolate bacteria. Isolation of Clostridium spp, Salmonella spp, and E. coli followed the methodology described in Brasil (2003).
Results
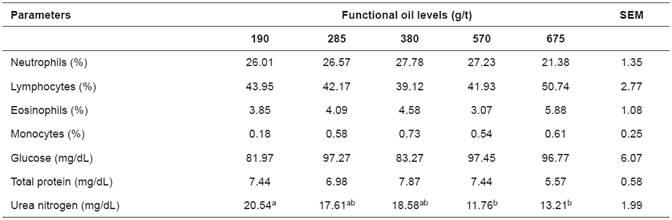
Functional oil levels did not influence WCC, glucose levels, or TP (p>0.05). Inclusion of 570 and 675 g/t FO resulted in lower BUN levels (p<0.05; Table 1).
Functional oils did not influence the presence of Clostridium sp nor E. coli in feces (p>0.05; Table 2); Clostridium sp and E. coli were present in all the samples. However, Salmonella spp was only found in the sheep fed 190 g/t FO.
Discussion
White cell count was not influenced, indicating that treatments did not affect the immune system of the sheep. White cell counts were within the normal reference levels for sheep (Meyer and Harvey, 2004).
Glucose was not affected by FO levels. Glucose results, between 81.97 and 97.45 mg/dL, were above the reference values reported by Meyer and Harvey (2004). Functional oils act in the rumen by changing ion transport in the cellular membrane of bacteria (Calsamiglia et al., 2007). They select for gram-negative bacteria and increase propionicacid (di Lorenzo, 2011) that is transformed into glucose by gluconeogenesis in the liver.
Table 1 White blood cell count (WCC) and levels of glucose, total protein (TP), and blood urea nitrogen (BUN) in sheep fed diets containing functional oils.
Different superscript letters (a, b) between columns indicate significant difference (p<0.05). SEM: Standard error of the mean.
Table 2 Bacterial presence in feces of sheep fed diets containing functional oils.

*Number of samples with bacterial growth/total number of samples.
Regarding total protein in blood, values were similar to those described by Kaneko (1997) and Contreras (2000). Thus, it can be inferred that inclusion of 190 g/t FO did not change the hepatic function and plasmatic protein production of the animals. Hypoproteinemia intensity is usually an indicator of the severity of gastrointestinal helminthiasis.
Blood urea nitrogen results were within reference values (17.12 to 42.8 mg/dL) published by Meyer and Harvey (2004). Plasmatic concentration of urea is positively correlated with intake of nitrogenous compounds (Valadares et al., 1999). Protein catabolism and its excretion rate depend on glomerular filtration rate and its reabsorption in renal tubules (Kaneko et al., 1997). Blood urea nitrogen is related to the efficiency of use of dietary protein, so high blood BUN concentrations could indicate inefficiencies in the dietary usage of protein, and also that high amounts of energy are being lost (Pessoa et al., 2009).
No difference was observed in fecal egg and oocyte numbers among treatments; all fecal samples contained E. coli and Clostridium spp colonies, but only samples from animals treated with 190 g/t FO tested positive for Salmonella spp. This indicates that only higher doses of FO can inhibit certain bacteria, as noted by Kubo et al. (1993). Ruminants are born with sterile gastrointestinal tracts, but it becomes colonized with several types of bacteria within a few hours after birth, including species of Lactobacillus,
E. coli and anaerobic bacteria, such as Clostridium and others (Quinn et al., 2012). Frias and Kozusny (2013) assessed the microbiota of healthy sheep and noted the presence of E. coli and Clostridium sp. In conclusion, functional oils can be added to sheep diets at 570 g/t to reduce blood urea nitrogen and presence of Salmonella spp in the feces.














