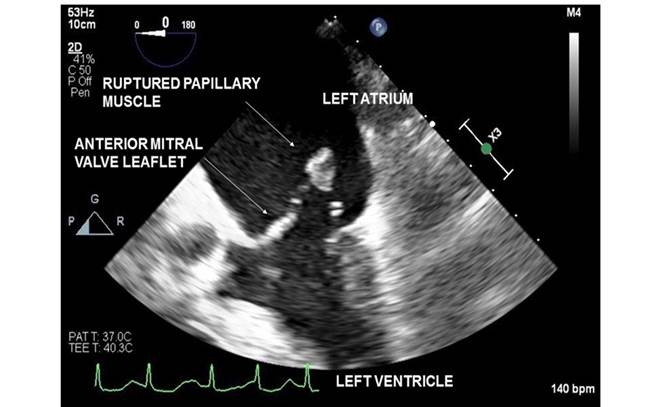
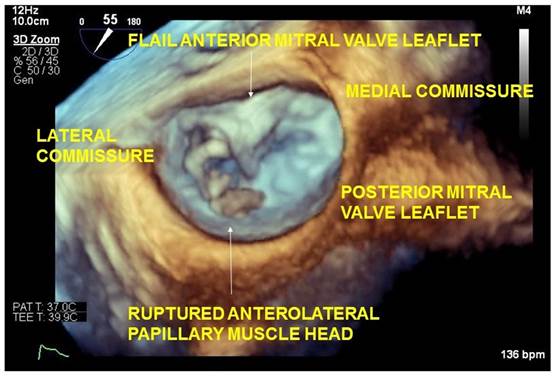
Left ventricular papillary muscle rupture is rare and may result from myocardial infarction, endocarditis, coronary vasospasm, Takotsubo cardiomyopathy and blunt chest trauma. In contrast to the posteromedial papillary muscle, which is supplied only by the posterior descending artery, the anterolateral papillary muscle has dual arterial blood supply from the left anterior descending and left circumflex arteries. Consequently, anterolateral papillary muscle rupture is an uncommon occurrence in myocardial ischemia or infarction.1,2 The accompanying 2D transesophageal echocardiography image demonstrates a ruptured papillary muscle head in the left atrium (Figure 1). The anterolateral origin of the ruptured papillary muscle is best confirmed with 3D imaging (Figure 2) or visualization of the rupture site in Transgastric 2 chamber view.
Source: Authors.
Figure 1 Transesophageal echocardiography image which demonstrates a ruptured papillary muscle head in the left atrium.
Source: Authors.
Figure 2 Anterolateral origin of the ruptured papillary muscle confirmed with 3D imaging.
Papillary muscle rupture leads to mitral valvular instability, loss of leaflet support and acute regurgitation. Anterolateral papillary muscle rupture leads to instability of the lateral hemivalve whereas posteromedial papillary muscle rupture results in medial hemivalve instability. In either condition, the left atrial and ventricular blood volumes increase suddenly giving the chambers no opportunity to dilate to accommodate the increased preload. Consequently, left atrial and pulmonary venous pressures increase markedly, often leading to pulmonary edema and hypoxemia. Additionally, effective stroke volume decreases as a large fraction of the left ventricular end diastolic volume is ejected into the left atrium in systole. This compromises cardiac output and systemic perfusion. Compensatory tachycardia and increased systemic vascular resistance from reflex neurohumoral responses worsen myocardial oxygen demand and potentiate myocardial ischemia. Clinical features include severe chest pain, dyspnea and precipitous hypotension. Infarction induced ventricular septal rupture and cardiac tamponade can present similarly necessitating use of echocardiography and invasive hemodynamic monitoring to guide decision making. In addition to vasoactive medications, mechanical circulatory support is often necessary. Left ventricular assist devices such as intra-aortic balloon counterpulsation pump or Impella help ameliorate cardiogenic shock by unloading the ventricle in systole, improving coronary perfusion and increasing cardiac index. Although mitral valve repair is possible, friable tissue makes the surgery technically challenging. Consequently, definitive therapy focuses on replacing the mitral valve. Cardiogenic shock and acute pulmonary edema make anesthetic management particularly challenging in these patients. Respiratory distress and hypoxemia related to pulmonary edema necessitates urgent intubation. However, tachypnea, inability to breathe deeply and alveolar edema related reduced functional residual capacity compromise pulmonary oxygen reserves and hamper effectiveness of preoxygenation. Additionally, inability to lie supine and pink frothy airway secretions make intubation challenging. In addition to establishing invasive arterial monitoring, central venous or pulmonary artery catheterization may be preferable prior to induction. Presence of cardiogenic shock necessitates judicious dosing of induction drugs and fluid administration. Surgical readiness should be confirmed prior to induction, in the event cardiac arrest necessitates emergent institution of cardiopulmonary bypass.2,3











 text in
text in 



