Introduction
Fluoride toothpastes are part of the category of topical, locally self-applied fluoride 1. Although toothbrushing with fluoride toothpaste is considered as: i) the most rational way of use of fluoride to control caries (2; ii) the reason for the caries prevalence decline observed in developed 3 and, developing 4 countries, and iii) the strongest way of fluoride use based on evidence 5, problems with toothpaste formulations have been described worldwide 4,6-13. According to the best available evidence 14, the minimum fluoride concentration that a toothpaste should have to display its maximum caries-protective effect is 1000 ppm (µg F/g), both for the primary 15 and permanent dentitions 5,16. In addition to interfere with the caries process − reducing demineralization and enhancing remineralization, the toothpaste’s fluoride the must be chemically soluble in the formulation 17.
Fluoride can be added into a toothpaste as sodium fluoride (NaF), stannous fluoride (SnF2), amine fluoride (AmF), or sodium monofluorphosphate (Na2PO3F = MFP). Due to chemical incompatibility leading to the formation of insoluble fluoride salts, toothpastes with NaF, SnF2 and AmF are not formulated with abrasives containing calcium 18,19, and therefore are usually formulated with silica. When the toothpaste’s formulation includes a calcium-based abrasive such as dicalcium phosphate dehydrate (DCPD) or calcium carbonate (CaCO3), MFP is used as the source of fluoride. Although toothpastes formulated with MFP do not form insoluble salts with calcium-based abrasives during its manufacturing process, the MFP molecule is hydrolyzed during toothpaste’s storage, releasing MFP ions that react with the calcium from the abrasive to form insoluble salts (insoluble fluoride, IF), reducing overtime the concentration of soluble caries-effective fluoride in the toothpaste 18.
For regulatory purposes toothpastes are considered cosmetics that are sold over the counter and in most countries the regulations only specify the maximum of total fluoride (TF) concentration that a toothpaste should have, without specifying how much of it is soluble 20. Countries of the Andean Region (Colombia, Venezuela, Ecuador, Perú, and Bolivia) follow the FDA regulatory parameters of toothpaste’s allowed ingredients 21. In Colombia, the National Institute for the Surveillance of Foods and Drugs (INVIMA) performs periodic sanitary surveillance of oral hygiene products, with the only requirement being to assure that the concentration of TF in toothpastes does not exceed the maximum limit of 0.15% F(1500 ppm F) 21.
Given that only the only toothpaste-fluoride concentration controlled by Colombian regulatory bodies is the TF, and there is a lack of knowledge on the caries-preventive chemically soluble fluoride in toothpastes sold in Colombia, this study aimed at testing the quality of the top-selling toothpastes brands sold in five representative cities of the country, assessing both the toothpastes’ TF and TSF fluoride concentrations.
Materials and methods
Experimental design
An exploratory study was conducted to assess the TF and TSF concentrations in the Colombian five top-selling toothpaste brands, one marketed for children: Colgate Smiles 6+ (A); and four marketed for family use: Kolynos Super Blanco (B), Fortident Cuatriacción (C), Colgate Triple Acción (D) and Fluocardent (E). Three tubes of each brand were bought in five cities representing the most populated regions of the country (n=3/brand/city): North region (Cartagena); Andean region (Medellín and Bogotá); East region (Cali), and West region (Villavicencio). Toothpastes were obtained from three different national chain stores, one tube per chain store in each city. All toothpastes were analyzed before their expiry date.
Determination of fluoride concentration
The analyses were made in duplicates following a standardized protocol 6,7. Briefly, an amount of 100 mg (± 10 mg) of each toothpaste was weighted, vigorously homogenized in 10 mL of deionized water, and 0.25 mL of this suspension were transferred in duplicates to new plastic assay tubes for TF analysis (TSF + IF). The remaining suspension was centrifuged (3,000×g/10 min, room temperature) to remove the insoluble fluoride bound to the abrasive (IF). The volumes of 0.25 ml of the supernatant were transferred in duplicate to new plastic assay tubes to determine fluoride ion’s (Fi) and TSF’s (F ion + MFP ion) concentrations. An amount of 0.25 mL of 2 M HCl were added to both tubes (TF and TSF) and after an incubation period of 1 h at 45, ºC the extracts were neutralized with 0.5 mL of 1 M NaOH and buffered with 1.0 mL of TISAB II. An amount of 1.0 ml of TISAB II, 0.5 mL of 1 M NaOH and 0.25 mL of 2 M HCl was added to the Fi tubes. The concentration of TSF determined with this protocol 6,7 was an indicator of bioavailable fluoride from in the MFP/CaCO3 toothpaste 21.
The concentration of fluoride ions was determined with an ion-selective electrode (Thermo Scientific Orion 96-09, Orion Research, Cambridge, MA, EUA) coupled to an ion analyzer Orion StarA214 (Orion Research, Cambridge, MA, EUA), previously calibrated with F standards having 0.0625 - 4.0 ppm F (μg F/ml) containing 0.25 M HCl, 0.25 M NaOH in TISAB II 50% (v/v). To determine the fluoride concentration in the samples (µg F/g) a linear equation was determined to explain the relationship between the logarithm of the fluoride concentration found in the standards and the mV readings displayed by the ion analyzer (r2 > 0.999). The variation coefficient of the repeated analyses (duplicate) was 1.3%. For data analyses, the means and standard deviations of the FT and FST concentrations found in each toothpaste brand sold in each city were calculated. In each sample, the IF concentration was calculated from the difference between FT and FST and expressed as the percentage of IF (% IF). The MFP ion was determined by the difference between the TSF and the Fi.
Statistical analyses
The percentage of disagreement between the measured and the declared TF was calculated by determining the proportion of the difference (TF declared - TF measured) that corresponds to the reference value (declared TF as 100%). Both percentage of disagreement and descriptive statistics (means and standard deviations) were calculated using Excel® (Microsoft Corporation, Redmond, WA, USA). A disagreement ≥ 5% was considered as non-compliance (6). Differences between TF and TSF concentrations, as well as the % IF, per brand, amongst the 5 different cities, were tested with ANOVA, using the software IBM SPSS Statistic 24 (IBM Corporation, Armonk, NY, USA). The significance level was set at 5% (α=0.05); thus, a p-value < 0.05 was considered to provide evidence of differences among the cities.
Results
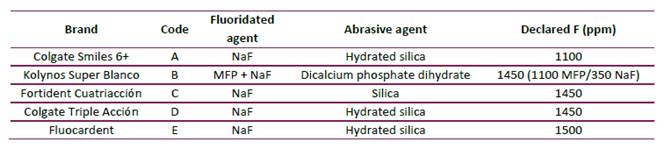
Table 1 shows that four out of the five toothpaste brands (A, C, D, E) analyzed are formulated with NaF as fluoride salt and silica-based abrasives, while only one brand (B) contains a combination of NaF and MFP as fluoride salts and a calcium-based abrasive. Additionally, all the brands included in this study had a concentration of total fluoride higher than 1000 ppm F, according to the information declared in the label by the manufacturer.
Table 1 Codification of the toothpaste brands selected, its fluoride salt, abrasive and declared fluoride concentration (ppm).
TF concentrations found were between 1068.4 and 1462.1 ppm F (Table 2) while those declared by the manufacturer in the toothpastes’ labels were between 1100 and 1500 ppm F. A disagreement higher than 5% amongst measured and declared TF in the toothpaste brands B and E (5.3% and 6.6%, respectively) was found, being the measured TF concentration lower than that declared (Table 2). All toothpastes showed TSF concentrations above the minimum required to exert a caries-protective effect (> 1000 ppm F) (Table 2).
Table 2 Total fluoride (TF), soluble fluoride (as fluoride ion, MFP ion and total fluoride) and percentage of insoluble fluoride (% FI) found in the top-selling toothpastes sold in five representative cities in Colombia (mean ± S.D., n=3).
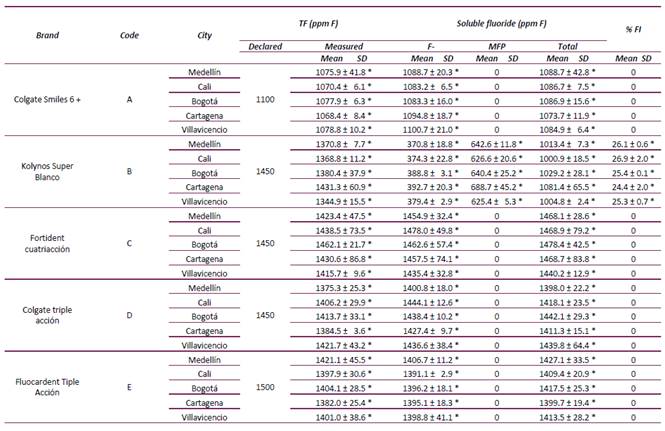
TF = Total fluoride; FI = insoluble fluoride
* Statistically significant differences among cities for each toothpaste and analysis (ANOVA, p < 0.05).
The TF and TSF concentrations of the same toothpaste’s brand, but purchased in different cities, were not statistically different (p > 0.05). Only one toothpaste had detectable levels of insoluble fluoride (brand B, 24.4-26.9% IF) with no statistically significant differences among the cities (p > 0.05) (Table 2).
Discussion
Most of the top-selling toothpaste brands sold in Colombia and analyzed in this study (A, C, D, E) contain NaF salt as source of fluoride and silica abrasive (Table 1), which are chemically compatible formulations. In this kind of formulation, all fluoride is potentially bioavailable to be delivered in the oral cavity during toothbrushing (7). On the other hand, toothpaste brand B was formulated with a mixture of MFP and NaF and contained dicalcium phosphate dihydrate as an abrasive. Besides the fact that there is no evidence that toothpaste formulations combining MFP and NaF are effective as anti-caries agents, this formulation may be less compatible that MFP/ dicalcium phosphate dihydrate-based or MFP/calcium-carbonate-based formulations.
All toothpastes analyzed showed a TF concentration that were very close to the concentration declared in the label (Table 2). None of them displayed TF concentrations > 1500 ppm F, the maximum value allowed by Colombian regulations 21. However, in terms of anti-caries benefits and dental fluorosis risk, it is relevant to know how much of the total fluoride is bioavailable, i.e. chemically available in the formulation to be released in the oral cavity during toothbrushing 7 or to have a systemic effect when ingested 7,22,23. Therefore, it is more important to know the total soluble fluoride (TSF) concentration contained in the toothpaste. The regulations followed by Colombia (a member of the Andean Region), fail in that they only establish the maximum TF concentration of the marketed toothpastes 21. According to the best available evidence, a toothpaste should contain 1000 ppm F or more to have caries-preventive efficacy 5,14-16 and is a fact that has been known for a long time 24,25. Currently, based on the mechanism of action of fluoride, it is known it has to be soluble in the toothpaste’s formulation 26. Very few countries have guidelines controlling toothpastes marketing based on their soluble fluoride concentration (20) and according to the World Dental Federation (FDI), a toothpaste should contain at least 800 ppm of soluble fluoride 27.
Our results show that all the toothpaste-brands evaluated in this study have a TSF concentration higher than 1000 ppm F before its expiry date, irrespective of the region of the city where they were bought (Table 2). Furthermore, in toothpastes A, C, D and E, all fluoride was soluble (TSF = TF), in agreement with their chemically compatible formulations (NaF/Silica-based). Among the analyzed toothpastes, the only exception (although not surprising), was brand B. In this toothpaste, the measured TSF concentration was lower than the TF concentration (Table 2); with the difference probably attributable to insoluble salts formed between the fluoride ion and the Ca from the abrasive. Furthermore, in brand B the fluoride ion was already present as NaF since its manufacturing and very likely due to MFP hydrolysis that occurs overtime during its storage. In toothpaste B, an average of 25.6% of the total fluoride was insoluble (Table 2); in other words, it is caries-preventive inactive because it is not bioavailable. Considering that it was analyzed from fresh samples, it is very likely that by the end of the expiry date, this brand will not maintain an effective fluoride anti-caries concentration, as it has already been shown for MFP/calcium carbonate 28. Therefore, it is advisable to evaluate this bran’s fluoride concentration’s stability over time.
Even though we have no exact data on the proportion of people using these specific brands in each city, the fact that these toothpastes are broadly distributed both in local and in national chain-stores, guarantees that these brands reach a significant portion of the population. The widely use of fluoridated toothpastes having a TSF concentration higher than 1000 ppm F has vary likely contributed to the decline in the prevalence of dental caries experience that has been observed in Colombia from the first to the fourth National Oral Health Study (conducted in 1966 and 2015, respectively) 29. However, it is of our knowledge that locally produced oral health-care products that are not approved, nor regulated, -and very likely not complying with regulations- are sold in the country, mainly in communities of low-socioeconomic status. Very little is known about the proportion of the population using such products and their impact in the prevalence of dental caries; thus, it would be interesting to survey the use of “non-conventional” toothpaste brands in Colombian communities where its marketing is suspected, to further analyze their quality.
Conclusions
Ttoothpaste brands highly distributed in five Colombian cities contain total fluoride concentrations that comply with Colombian regulations. Fresh samples from all toothpaste brands complied with the minimum chemically soluble fluoride concentration required for the prevention of dental caries, regardless of the purchase region. However, one toothpaste formulation containing NaF and dicalcium phosphate dehydrate as abrasive, had chemically soluble fluoride concentrations lower than the total fluoride, probably because of a non- chemically compatible formulation. The fluoride concentration in this toothpaste brand should be analyzed overtime to evaluate whether it maintains effective fluoride concentrations up to its expiry date.















