INTRODUCTION
Approximately 250 insect species attack crops, grains, and their products during storage. About twenty are of utmost importance (Manosathiyadevan et al., 2017). Among the most detrimental is the brown corn beetle Sitophilus zeamais Motschulsky, which receives its common name from the coloration and infestation habits in the grains of Zea mays L. This pest has its origin in Asia but is currently distributed throughout the world (Corrêa et al., 2017). Among the characteristics that define it as a pest of agricultural interest are its flight capacity, walking capacity, body mass, and grain consumption. The beetles feed on the embryo and thus reduce the germination percentage of the seeds (Choden et al., 2021). Larvae and adults cause the most significant damage to the grain, especially the females, since they oviposit inside the seed and seal it with a gelatinous secretion, allowing the complete development of the new individual inside the grain. For this reason, most of the strategies to control S. zeamais are carried out on adults (Nwosu, 2018). Pesticides based on synthetic compounds (e.g., organophosphates, pyrethroids, carbamates, etc.) are used to control and minimize pests in crops and stored products (Hamel et al., 2020). These chemical substances are released into the environment to eradicate, prevent, control, repel, or mitigate pests and weeds in agricultural, domestic, and industrial environments (Islam et al., 2022). However, these compounds can generate contamination of soil, water, air, and food, affecting biodiversity and ecosystems due to their toxicological characteristics (Nwosu, 2018). Additionally, they can cause acute or chronic intoxication in humans and animals exposed to or consuming these substances (Eijsackers and Maboeta, 2023). Some synthetic pesticides may be carcinogenic, mutagenic, teratogenic, or endocrine disruptors; this has led to their prohibition in some cases (Fernández et al., 2023).
Biopesticides appear as a more ecological and safer alternative for pest management (Bhavya et al., 2021; Chaudhari et al., 2021; Stejskal et al., 2021). Among these are those based on essential oils (EOs), which, in addition to presenting functional capacities like a synthetic pesticide, are biodegradable (Ram and Singh, 2021). EOs extracted from aromatic plants have long been of scientific relevance due to their biocidal properties against insects. They show insecticidal and fumigant actions against several pests of agronomic and medical importance, as well plant pathogens (Smith et al., 2018; Khursheed et al., 2022).
Around 6,500 plant species have been studied for their insecticidal properties. More than 2,500 belong to 235 families with biocidal activity (Giraldo-Rivera and Guerrero-Alvarez, 2019). Among the families with biopesticidal activity are the Piperaceae (Andrés et al., 2017; Jaramillo-Colorado et al., 2019; de Souza et al., 2020; Le et al., 2022). However, the insecticidal activity of the EOs of this family has been little evaluated in S. zeamais (Peschiutta et al., 2022). The Piperaceae family comprises approximately five genders and more than 3,000 species. The genus Piper L. is the largest of this family and the second largest in the world, with 2,171 species (Amorim et al., 2023), and has a cosmopolitan distribution in tropical and subtropical regions (Jaramillo and Manos, 2001). This genus has most of its diversity in humid forests, mountains, and lowlands. They are small trees, shrubs, ivies, and in smaller proportion, hemiepiphytes. The leaves are simple, lanceolate, and alternate; the thick stems and the flowers are arranged in peduncular or erect inflorescences (Jaramillo et al., 2004). Approximately 1,900 taxa are distributed in tropical America (Amorim et al., 2023), and only about 50 species have been studied for biocidal activity (Carmona-Hernández et al., 2016). In terms of chemical components, the species of the Piper genus can present terpenes (piperitone, linalool, selinene), propenylphenols (apiol, safrole), amides (piperidine), among others (Whitehead et al., 2013; Chandra et al., 2014; Silva et al., 2019). These components are potential biopesticides against pests. Some compounds identified in this study are shown in figure 1.
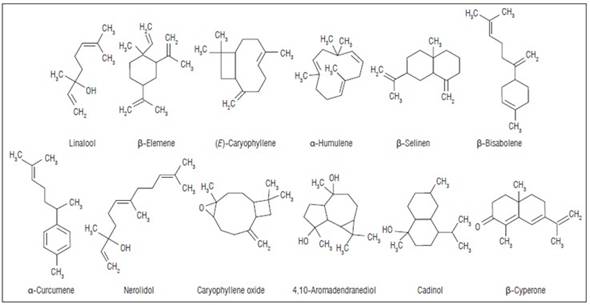
Figure 1. Chemical structures of the main compounds found in the EOs of P. coruscans, P. ottoniaefolium, and P. reticulatum. Developed using MarvinSketch ver. 23.2.
To date, for P. ottoniaefolium, P. reticulatum and P. coruscans, no biological activity against the S. zeamais has been reported, but biological activity against some fungi, insects and parasites has been documented (Santana et al., 2016; Whitehead and Bowers, 2014; Ruiz-Vásquez et al., 2022; Vásquez-Ocmín et al., 2022). For these reasons, the present research seeks to determine the biocidal activity of the EOs to P. ottoniaefolium, P. reticulatum and P. coruscans, obtained from the Department of Chocó, Colombia, and thus promote their potential use as insecticidal agents against S. zeamais.
MATERIALS AND METHODS
Plant material
Specimens of the three Piperaceae were collected in March 2022 from localities in the department of Chocó (Colombia). Specimens of P. coruscans were obtained from the municipality of Garcia Gomez (4°10'0.012" N, 77°25'0.011" W), P. ottoniaefolium from the vicinity of the Universidad Tecnológica del Chocó (5°40'54" N, 5°3'49" W) and P. reticulatum from the municipality of Lloro (5°36'4" N, 76°22'26" W). One specimen of each species was deposited in the National Herbarium of Colombia (COL) with voucher codes 552836 (P. coruscans), 522551 (P. ottoniaefolium), and 514401 (P. reticulatum).
Essential oils extraction
The essential oils were extracted by the hydrodistillation (HD) technique using a Clevenger-type apparatus, as described by Jaramillo et al. (2012). 500 g of finely chopped fresh leaves and distilled water (2-3 L) were added to a flask (5 L). The extraction time was 2-3 h. The EOs obtained were collected in vials (5 mL) and anhydrous sodium sulfate (Na2SO4) was added to remove traces of water. Finally, the EOs were transferred to new vials, where the extraction yield was determined by the equation:
Gas chromatography analysis
Chromatographic analysis was performed on an Agilent 7890A series gas chromatograph coupled to an Agilent MSD 5975C mass spectrometry detector (GC-MS), using an HP-5MS capillary column (350°C, 30 m × 250 µm id × 0.25 µm pd), following the methodology proposed by Adams (2017), with some modifications. GC-MS was used under the following conditions: 1 μL of the diluted 1% v/v EOs in dichloromethane was injected in split-less mode (230°C). The initial oven temperature was 50°C for 2 min and a ramp of 3°C/min up to 250°C was programmed. The carrier gas used was He, with a pressure of 12.6 psi at a flow rate of 1 mL min-1. Mass spectra were obtained by electron impact ionization at 70 eV energy, with a mass acquisition range of 30-700 m/z.The identification of the constituents of the chemical composition was performed by comparing the mass spectra obtained with the spectra available in the NIST08 library databases, and the Kovats retention indices were determined by comparing with the retention times obtained from an internal standard of n-alkanes (C7-C40), under the same analysis conditions.
Growth and identification of target insects
Adult specimens of S. zeamais were obtained from grain stored in a local market and taxonomically identified, according to Rees (2007). Insect rearing was carried out at the Agrochemical Research Laboratory, University of Cartagena, in 4 L glass jars with a diet of 1.5 kg corn and 500 g barley. The conditions were maintained at 30±2°C, 70% relative humidity, and 12:12 (D/N) photoperiods.
Bioassays insecticidal activity
Repellent activity
The EOs of P. coruscans, P. ottoniaefolium, P. reticulatum, and a commercial repellent, Stay Off® (3-(N-acetyl-N-butylamino) ethyl propionate, 15%) were evaluated on 7-10 d old, unsexed adult weevils. In addition, two compounds, caryophyllene oxide (Sigma-Aldrich, 95%) and linalool (Merck KGaA, 97%), were studied. This activity was based on the preference area method, according to Jaramillo-Colorado et al. (2014) with some modifications. In the interior of 9 cm Petri dishes, discs of Whatman® N°1 filter paper of 9 cm in diameter cut in half were placed, resulting in two working areas. To each half of the paper, 100 μL of different concentrations of the EOs, the standards, and the commercial product dissolved in acetone were added (0.31, 0.62, 1.25 and 2.50 μg cm-2). The other half of the paper was used as a control, with the addition of acetone. The paper disks were dried in a fume hood for 10 min to evaporate the solvent. After this time, 20 adult specimens of S. zeamais were added to the center of the petri dishes. These were then covered and sealed with kerosene paper (Parafilm®) to prevent the escape of the insects. The assay was carried out in total darkness. Finally, after 2, 4, and 6 h of exposure time, the number of individuals present in the treated and untreated areas was counted. The percentage of repellency (%PR) was calculated by %PR = [(Nc - Nt) / (Nc + Nt) x 100], where Nc is the number of insects present in the control and Nt the number of insects in the treated area. Each treatment was performed in triplicate and the experiment twice.
Fumigation activity
The toxic effect of P. coruscans, P. ottoniaefolium, P. reticulatum, and D-WT (Chlorpyrifos, 44%) were evaluated on a sample of 20 unsexed S. zeamais specimens, aged 7 to 10 d, following the methodology described by Brito et al. (2021). Additionally, two compounds were tested: caryophyllene oxide (Sigma-Aldrich, 95%) and linalool (Merck KGaA, 97%). Filter paper discs (Whatman® N° 1, 2.4 cm diameter) treated with 150 μL of the different concentrations of each treatment in acetone (2.5, 5.0, 10, and 20 μL cm-3 air) were placed in 20 cm3 screw cap vials. The filter paper discs were dried in an extraction hood for 15 min. Subsequently, they were introduced into the vials supporting them on the lids to avoid contact with the weevils. Acetone was used as a control. The test was carried out in complete darkness, and after 24 h, the number of dead individuals was counted (those that did not show movement of legs or antennae). Each treatment was carried out in triplicate. The results were used to calculate the percentage of mortality (%M), which was corrected with Abbott’s equation (1925): %M = [(%Mt - %Mc) / (100 - %Mc)]*100, where %Mt is mortality with the treatment and %Mc mortality with the control. Finally, LC50 and LC95 were calculated for each treatment.
Statistical analysis
A multifactorial analysis of variance was performed to determine significant interactions between concentration and time in the repellency bioassays to verify whether the percentage of repellency (%PR) depended on the concentration or the exposure time. The repellent effect of the EO was compared with the commercial product by t-test (P<0.05). From the results obtained in the insecticidal activity, LC50 and LC95 values were determined by linear regression of Probit analysis with parameters of P<0.05, indicating a confidence level of 95%. In addition, the insecticidal effects of the EOs against the commercial product were compared by ANOVA, followed by a least squares mortality adjustment. Means were compared by a Tukey test (P<0.05). All statistical analyses were performed using Statgraphics Centurion® v.19 software.
RESULTS AND DISCUSSION
Extraction yield
Of the three Piper species used in this study, the highest amount of essential oil was obtained from P. ottoniaefolium, while hydrodistillation of P. coruscans produced the lowest yield (Tab. 1). The plant material of P. reticulatum yielded more essential oil than P. coruscans and lower than P. ottoniaefolium; however, it recorded the highest density of the three oils (Tab. 1).
Table 1. Yields and characteristics of essential oils (EOs) from Piper coruscans, P. ottoniaefolium and P. reticulatum.
| EO | Yield (%) | Density (g mL-1) | Color |
|---|---|---|---|
| P. coruscans | 0.32±0.01 | 0.86±0.03 | Light yellow |
| P. ottoniaefolium | 0.47±0.02 | 0.84±0.01 | Light yellow |
| P. reticulatum | 0.38±0.02 | 0.92±0.01 | Dark yellow |
Average±standard deviation.
Gas chromatography analysis
The main compounds of the EOs of the three Piperaceae are shown in table 2. For P. coruscans, 19 compounds were identified, with the major components being caryophyllene oxide (31.75%), β-selinene (10.29%), β-caryophyllene (7.12%), humulene epoxide II (6.07%), and β-elemene (5.57%). In P. ottoniaefolium, 45 compounds were identified. The principal compounds found were β-bisabolene (14.46%), α-curcumene (8.36%), 4,10-aromadendranediol (7.86%), aromadendrene (5.46%), and α-bisabolene epoxide (5.44%). Finally, in P. reticulatum, 63 compounds were identified, with caryophyllene oxide (9.44%), β-caryophyllene (9.01%), β-selinene (5.15%), α-copaene (5.05%), and β-cyperone (4.69%) as the main ones.
Table 2. Volatile chemical composition of essential oils from Piper coruscans (Pco), P. ottoniaefolium (Pot) and P. reticulatum (Pre) by GC-MS.
| Compounds | Relative area (%) | IKa | ||
|---|---|---|---|---|
| Pco | Pot | Pre | ||
| α-Pinene | 1.50 | - | - | 939 |
| 3-Carene | - | - | 0.72 | 1,008 |
| p-Cymene | - | 1.78 | 0.29 | 1,024 |
| β-Terpinene | 1.65 | - | - | 1,054 |
| Acetophenone | - | 0.78 | - | 1,059 |
| Linalool | 3.29 | 0.84 | 2.49 | 1,096 |
| 1,3,8-p-Menthatriene | - | 0.91 | - | 1,108 |
| trans-Pinocarveol | - | - | 0.31 | 1,135 |
| D-Limonene | 1.61 | 0.84 | 0.35 | 1,136 |
| trans-Verbenol | - | 2.41 | - | 1,140 |
| Menthone | - | 0.79 | 0.60 | 1,148 |
| α-Terpineol | - | - | 0.64 | 1,186 |
| Myrtenol | - | - | 0.35 | 1,194 |
| Benzylacetone | - | 0.79 | - | 1,228 |
| Piperitone | 1.43 | - | 2.48 | 1,249 |
| Safrole | - | - | 0.32 | 1,285 |
| 3-Undecanol | - | - | 1.90 | 1,293 |
| Panaxene | - | - | 1.93 | 1,312 |
| α-Cubebene | - | 0.94 | 3.28 | 1,351 |
| α-Ylangene | - | 0.78 | - | 1,373 |
| α-Copaene | 1.78 | 1.20 | 5.05 | 1,374 |
| β-Cubebene | - | 0.81 | - | 1,387 |
| β-Bourbonene | - | 1.08 | 0.35 | 1,388 |
| Isolongifolene | 2.39 | - | 0.55 | 1,389 |
| β-Elemene | 5.57 | 0.83 | 3.11 | 1,390 |
| α-Funebrene | - | - | 0.30 | 1,402 |
| β-Caryophyllene | 7.12 | 2.25 | 9.01 | 1,419 |
| α-Ionone | - | - | 0.38 | 1,428 |
| β-Copaene | - | - | 0.38 | 1,430 |
| β-Gurjunene | - | - | 0.31 | 1,431 |
| cis-Thujopsene | - | - | 0.75 | 1,431 |
| trans-α-Bergamotene | - | 0.80 | 0.32 | 1,432 |
| α-Humullene | 3.28 | 1.03 | - | 1,438 |
| β-Famesene | - | 1.58 | - | 1,443 |
| cis-muurola-3,5-diene | - | - | 0.33 | 1,448 |
| α-himachalene | - | 0.85 | - | 1,449 |
| (E)-Cinnamic acid | 3.04 | - | - | 1,452 |
| α-Neoclovene | - | 1.72 | - | 1,452 |
| Alloaromadendrene | - | - | 0.32 | 1,461 |
| 9-epi-(E)-Caryophyllene | - | - | 1.75 | 1,466 |
| β-Acoradiene | - | - | 0.29 | 1,469 |
| α-Terpinyl isobutanoate | - | - | 0.48 | 1,471 |
| α-Elemene | - | - | 3.33 | 1,477 |
| γ-Muurolene | - | 2.25 | 0.60 | 1,479 |
| Germacrene D | 3.87 | 4.68 | 2.00 | 1,481 |
| β-Selinene | 10.29 | - | 5.15 | 1,490 |
| epi-Cubebol | - | 1.08 | 0.70 | 1494 |
| Viridiflorene | - | - | 0.78 | 1,496 |
| α-Selinene | - | 1.02 | - | 1,498 |
| α-Muurolene | - | 1.34 | 0.48 | 1,500 |
| Cuparene | - | - | 1.45 | 1,504 |
| β-Bisabolene | - | 14.46 | - | 1,505 |
| γ-Cadinene | 1.50 | 0.92 | - | 1,513 |
| α-curcumene | 8.36 | 3.17 | 1,515 | |
| β-Sesquiphellandrene | - | 2.68 | 0.33 | 1,522 |
| trans-Calamenene | - | - | 0.86 | 1,522 |
| Nerolidol | 2.88 | 1.20 | 0.48 | 1,532 |
| α-Calacorene | - | 0.98 | 0.76 | 1,545 |
| Elemol | 0.60 | 1,549 | ||
| Caryophyllene oxide | 31.75 | 3.88 | 9.44 | 1583 |
| Boronal | - | - | 0.85 | 1,585 |
| Salvial-4(14)-en-1-one | - | 1.38 | 0.70 | 1,595 |
| Widdrol | - | 1.03 | - | 1,599 |
| Ledol | - | - | 0.38 | 1,602 |
| Humulene epoxide II | 6.07 | - | 3.23 | 1,608 |
| Zingiberenol | - | 1.13 | - | 1,626 |
| Aromadendrene-4,10-diol | - | 7.86 | - | 1,639 |
| τ-Muurolol | - | - | 2.51 | 1,642 |
| β-Eudesmol | - | - | 0.42 | 1,649 |
| τ-Cadinol | - | 1.56 | 0.76 | 1,650 |
| α-Cadinol | - | 1.59 | - | 1,654 |
| Neointermedeol | 4.63 | - | - | 1,660 |
| Cadalene | - | - | 0.82 | 1,675 |
| trans-Sesquisabinene hydrate | - | - | 0.59 | 1,677 |
| Apiole | - | - | 1.11 | 1,678 |
| Aromadendrene | 5.46 | 0.43 | 1,680 | |
| Shyobunol | - | - | 0.41 | 1,688 |
| cis-Thujopsenal | - | - | 0.68 | 1,709 |
| α-Cyperone | 4.57 | - | - | 1,727 |
| Isolongifolol | - | 3.07 | 3.99 | 1,729 |
| β-Cyperone | - | - | 4.69 | 1,746 |
| Cuparenal | - | 1.36 | - | 1,753 |
| α-Costol | - | 1.41 | - | 1,774 |
| α-bisabolene epoxide | - | 5.44 | 3.36 | 1,814 |
| Hexahydrofarnesyl acetone | - | 1.69 | - | 1,834 |
| Longifolenaldehyde | - | - | 1.88 | 1,876 |
| (8S,14)-Cedrandiol | - | - | 0.57 | 1,889 |
| Phytol | - | 0.92 | 2.47 | 1,943 |
| Oxygenated hydrocarbons | 3.04 | 1.57 | 1.90 | |
| Oxygenated monoterpenes | 4.72 | 4.04 | 7.19 | |
| Oxygenated sesquiterpenes | 49.9 | 34.6 | 39.14 | |
| Monoterpenes | 4.76 | 3.53 | 1.74 | |
| Sesquiterpenes | 35.80 | 56.02 | 49.35 | |
| Total | 98.22 | 99.76 | 99.32 | |
a Experimentally determined Kováts indices in a HP-5 column.
The EOs of the three Piper species are often classified in five groups (Da Silva et al., 2017): predominantly monoterpenes (piperitone, α-pinene), sesquiterpenes (β-selinene, β-caryophyllene, β-elemene, aromadendrene, β-bisabolene, α-curcumene, β-cyperone, α-copaene), phenylpropanoids (safrole, apiole), sesquiterpene alcohols (4,10-aromadendranediol, isolongifololol), and oxygenated terpenoids (caryophyllene oxide, humulene epoxide II, α-bisabolene epoxide).
The EOs of three Piperaceaestudied showed qualitative variations in contrast to oils from plants collected in other regions of tropical America. In the EO of P. coruscans collected in the Peruvian Amazon, the main components obtained were β-bisabolene (33.4%), and nerolidol (10.2%) (Ruiz-Vásquez et al., 2022). Gilardoni et al. (2020) reported for P. coruscans native to the coast and Amazon of Ecuador, (E)-β-caryophyllene (24.1%), α-humulene (11.6%), caryophyllene oxide (9.3%) and linalool (4.5%) as the major compounds in the leaves of this plant. The EO of P. reticulatum leaves collected in the Peruvian Amazon contained apiol (15.0%) and D-germacrene (12.6%)(Ruiz-Vásquez et al., 2022). In comparison, EO from a Brazilian region was characterized by β-elemene (24.6%) followed by β-caryophyllene (16.7%) (Luz et al., 2003) as principal compounds. This last compound coincides with the second principal compound reported in this research for P. reticulatum. Regarding the EO of P. ottoniaefolium, no reports of the volatile chemical composition of this oil were found, confirming that this research is the first to describe the components of its EOs. The main compound in this oil was β-bisabolene, a sesquiterpene found in the EO of various plants, including those of the genus Piper. These results coincide with the EO of Piper lepturum var. angustifolium, where β-bisabolene (17.72%) was the predominant compound, and in Piper coruscans was β-bisabolene, (33.4%) (Salehi et al., 2019; Ruiz-Vásquez et al., 2022).
The yield and chemical composition of essential oils can present chemical variations due to exogenous factors such as temperature, soil, light, rainfall, place of cultivation, and predators (Figueiredo et al., 2008; Karimi et al., 2020), well as endogenous factors, such as volatile compound biosynthesis pathways and anatomical and physiological differences in plants (Barra, 2009).
Repellent activity
The results of the repellent activity of the essential oils from the Piper species tested, and the commercial product, Stay Off® ((3-(N-acetyl-N-butylamino)), against S. zeamais are shown in table 3. The three essential oils showed the highest percentage of repellency at 2.5 μg cm-2 after 2, 4 and 6 h of exposure: P. coruscans (66.7±0.06%, 56.7±0.06%, 3.3±0.06%), P. ottoniaefolium (83.3±0.06%, 43.3±0.15%, -10.0±0.10%), and P. reticulatum (36.7±0.12%, 33.3±0.21%, -3.3±0.59%), respectively. Positive values indicate repellent activity, and negative values indicate attractive activity. For this reason, at 6 h of exposure, the EOs of P. ottoniaefolium and P. reticulatum showed attractive activity, and P. coruscans EOwas the only one that showed repellent activity. The Stay Off® showed the highest percentage of repellency at 2, 4 and 6 h of exposure to 2.5 μg cm-2 (63.3±0.1%, 37.8±0.0%, -3.3±0.1%), respectively. Linalool showed a repellent effect at 2, 4, and 6 h of exposure at 2.5 μg cm-2, with the highest percentage of repellency for this compound obtained at 2, 4 and 6 h of exposure (60.0±0.00%, 53.3±0.12%, 20.0±0.10%) and 1.25 μg cm-2 (13.3±0.64%), respectively. Caryophyllene oxide did not show repellent activity under any concentration at the exposure times tested.
Table 3. Repellent activity of essential oils from Piper coruscans, P. ottoniaefolium and P. reticulatum, linalool, caryophyllene oxide and Stay off ® (commercial control) against Sitophilus zeamais.
| Compounds | Concentration (μg cm-2) | Percentage of repellency, according to exposure time (hours) | ||
|---|---|---|---|---|
| 2 | 4 | 6 | ||
| P. coruscans | 2.50 | 66.7±0.06 a | 56.7±0.06 | 3.3±0.25 |
| 1.25 | 36.7±0.06 a | 13.3±0.12 | -16.7±0.15 | |
| 0.62 | 16.7±0.06 a | 3.3±0.12 | -36.7±0.12 | |
| 0.31 | 3.3±0.06 a | -6.7±0.12 | -43.3±0.32 | |
| P. ottoniaefolium | 2.50 | 83.3±0.06 | 43.3±0.06 | -10.0±0.10 |
| 1.25 | 46.7±0.06 a | 33.3±0.12 | -20.0±0.20 | |
| 0.62 | 43.3±0.15 a | 0.0±0.10 | -26.7±0.12 | |
| 0.31 | 13.3±0.12 a | -6.7±0.15 | -33.3±0.15 | |
| P. reticulatum | 2.50 | 36.7±0.12 a | 33.3±0.21 | -3.3±0.59 |
| 1.25 | 26.7±0.12 a | 16.7±0.45 | -26.7±0.38 | |
| 0.62 | 13.3±0.06 a | 3.3±0.21 | -36.7±0.38 | |
| 0.31 | 10.0±0.10 a | -13.3±0.31 | -50.0±0.10 | |
| Linalool | 2.50 | 60.0±0.00 a | 53.3±0.12 | 20.0±0.10 |
| 1.25 | 30.0±0.10 a | 23.3±0.55 | 13.3±0.64 | |
| 0.62 | 33.3±0.15 a | 6.7±0.06 | -10.0±0.10 | |
| 0.31 | 20.0±0.00 a | -6.7±0.12 | -23.3±0.21 | |
| Caryophyllene oxide | 2.50 | -3.5±0.18 | -18.3±0.14 | -21.3±0.10 |
| 1.25 | -8.7±0.13 | -24.1±0.03 | -30.7±0.32 | |
| 0.62 | -14.3±0.21 | -37.2±0.12 | -49.8±0.28 | |
| 0.31 | -28.6±0.16 | -44.2±0.31 | -59.1±0.26 | |
| Stay off | 2.50 | 63.3±0.1 | 37.8±0.0 | -3.3±0.1 |
| 1.25 | 30.0±0.1 | 5.5±0.1 | -23.3±0.2 | |
| 0.62 | 3.3±0.1 | -13.3±0.1 | -20.0±0.1 | |
| 0.31 | -16.7±0.2 | -46.7±0.2 | -11.6±0.2 | |
aThere is a statistically significant difference between the essential oil and the commercial repellent (P<0.05). Mean±standard deviation.
The statistical analysis of the repellent activity, through the comparison of means by analysis of variance, for P<0.05, showed significant differences at 2 h of exposure for P. coruscans (0.00002), P. ottoniaefolium (0.00048), P. reticulatum (0.00193), and linalool (0.00212), compared to the commercial product Stay Off®. In contrast, at 4 and 6 h of exposure, no statistically significant differences were observed for P. coruscans (0.10432, 0.732014), P. ottoniaefolium (0.08093, 0.68728), P. reticulatum (0.11431, 0.33144), and linalool (0.10267, 0.20447), respectively.
The confidence intervals at 2, 4 and 6 h of exposure for P. coruscans were:[0.32503; 0.74163], [-0.04667; 0.46334], [-0.12448; 0.17448]; for P. ottoniaefolium: [0.19697; 0.60302], [-0.02896; 0.46229], [-0.15210; 0.10210]; for P. reticulatum: [0.16430; 0.63569], [-0.05659; 0.48992], [-0.10882; 0.30882] and linalool: [0.1684; 0.66493], [-0.05083; 0.51750], [-0.08300; 0.36633], respectively. Since, at 2 h of exposure in all treatments, the intervals for all treatments do not contain the value 0, there are statistically significant differences between the means of the treatments with Stay Off®with a confidence level of 95%, in contrast to the case seen at 4 and 6 h of exposure, where the value 0 is included in the confidence intervals. From the above, it is assumed that there are no significant differences between the means compared. Considering these results, the EOs of P. coruscans, P. ottoniaefolium, P. reticulatum and linalool did not show similarity with Stay off® at 2 h. From this, it can be indicated that the EOs have a repellent activity superior to the commercial repellent on S. zeamais.
The results obtained in this research show a high repellent activity of Piper EO against S. zeamais. This is consistent with published studies on the repellent effects of EOs from Piperaceae against several pests (Araújo et al., 2012; Vasantha-Srinivasan et al., 2018; Muñoz-Acevedo et al., 2023). Of the three tested EOs, P. ottoniaefolium showed a higher repellency percentage (83.3±0.06%) than P. coruscans (66.7±0.06%) and P. reticulatum (36.7±0.12%), at 2.5 μg cm-2, after 2 h of exposure, on S. zeamais. This could be explained by the chemical composition of P. ottoniaefolium EO, of which β-bisabolene (a sesquiterpene) was identified as the primary compound. Previous studies have reported repellent potential in sesquiterpenes (Fraga et al., 2021; Jiménez-Durán et al., 2021). Wei et al. (2018) showed a high repellent effect of β-bisabolene against the cigarette beetle, Lasioderma serricorne Fabricius, and the booklouse Liposcelis bostrychophila Badonnel. However, after 4 and 6 h of exposure P. coruscans had the highest stability in repellent activity, being the only EO to show repellency at 2.5 μg cm-2 and 6 h (3.3±0.25%) in contrast to the EOs of P. ottoniaefolium (-20.0±0.20%) and P. reticulatum (-3.3±0.59%) which showed attractive activity at all concentrations evaluated at the latter exposure time. This is one of the reasons why the effects are so differentiated as the exposure time elapses (Chan et al., 2016; Kaur et al., 2021; Zhang et al., 2023). It is not possible to affirm that the repellent activity of EOs against insects is limited only to the potential of some of their main constituents, since this effect could also be due to certain minority compounds or the antagonistic or synergistic behavior of several compounds (Dhifi et al., 2016; Dassanayake et al., 2021; Kaur et al., 2021).
The linalool standard (terpenoid alcohol) showed the highest percentage of repellency at 2.5 μg cm-2 after 2 h of exposure (60.0±0.00%). These results agree with those reported by Karemu et al. (2013), where this compound obtained a repellency of 63.3% at the highest dose tested (2.0 μg μL-1). This compound has also been evaluated on several species of mosquitoes, resulting in high repellency rates (Müller et al., 2009; Dekker et al., 2011; Murtaza et al., 2023) and in beetles, lice, and flies (Yang et al., 2014; Wang et al., 2019; Papanastasiou et al., 2020).
Fumigant activity
The results of the percentage of mortality of the EOs of P. coruscans, P. ottoniaefolium,andP. reticulatum are shown in figure 2. The oils of P. coruscans, and P. ottoniaefolium reached mortality of 100% in 20 µL cm-3 air. In comparison, the oil of P. reticulatum at the same concentration showed a mortality of 73.3±0.31%. The tested substances: linalool, caryophyllene oxide, and the commercial insecticide D-WT (chlorpyrifos), showed mortalities of 96.7±2.87, 13.4±3.77, and 98.3±2.88%, in 20 µL cm-3, respectively. At the lowest concentration tested, 2.5 µL cm-3 air, the insecticidal activity of the EOs was: P. coruscans, 15.0±0.05%, P. ottoniaefolium, 23.9±0.10%, and P. reticulatum, 23.8±0.08%. Similarly, linalool, caryophyllene oxide, and the commercial insecticide reached mortalities of 50.1±5.03, 0, and 86.7±7.63%, respectively. Based on the above and considering that the mortality of the standards is higher than that of the EOs studied, they are considered fumigants against S. zeamais; they must be applied in low doses to show their biocidal capacity, due to the physicochemical properties of these compounds (Wang et al., 2021).

Figure 2. Mortality rate of Sitophilus zeamais at different concentrations of the essential oils Piper coruscans (A), P. ottoniaefolium (B) and P. reticulatum (C), the standards caryophyllene oxide (D) and linalool (E), and the commercial product D-WT chlorpyrifos (F) after 24 h of exposure.
The toxicity of EOs against insects is due to the rapid action of the principal metabolites present in their composition (Angane et al., 2022). This toxicity is generated by four routes: inhalation, contact, absorption, and ingestion. Similarly, several mechanisms of toxic action of EOs against insects have been described, such as inhibition of acetylcholinesterase, blocking of gamma-aminobutyric acid (GABA) channels, and interference with the neuromodulator octopamine, among others (Koyama and Heinbockel, 2020; Hung et al., 2022; Mattar et al., 2022; Mssillou et al., 2022). It has also been confirmed that the minority components in EOs can also affect these mechanisms because they exert synergistic actions with the other compounds present in EOs (Yuan et al., 2019; Kim et al., 2021).
Table 4 shows the LC50 and LC95 values against S. zeamais for the EOs P. coruscans, P. ottoniaefolium,and P. reticulatum, the substances linalool, caryophyllene oxide and the commercial insecticide D-WT (chlorpyrifos) after 24 h of exposure. The results of the Probit analysis showed that the EOs obtained LC50, values in µL cm-3 air of 7.43 (P. coruscans) 3.56 (P. ottoniaefolium) and 6.12 (P. reticulatum), and LC95 values in µL cm-3 air of 16.07, 5.95 and 28.75, respectively. When the analysis of variance was performed for the comparison of means by Tukey's test, with an P<0.05, statistically significant differences were obtained between the insecticidal capacities of the EOs and linalool against the commercial insecticide DW-T. The p values obtained from the comparisons were P. coruscans (0.0002), P. ottoniaefolium (0.0060), P. reticulatum (0.0012)and linalool (0.0361). The 95.0% confidence intervals for the mean difference were P. coruscans [0.4171, 0.6296], P. ottoniaefolium [0.5413, 0.7537], P. reticulatum [0.4637, 0.6762] and linalool [0.6729, 0.8854]. Since the intervals do not contain the value 0, the existence of a statistically significant difference between the means of mortality of EOs and linalool against DW-T is reaffirmed. This allows estimating that the insecticidal capacity of the oils and linalool are not comparable to the commercial product.
Table 4. Toxicity of the essential oils Piper coruscans, P. ottoniaefolium and P. reticulatum, the commercial substances linalool, caryophyllene oxide and D-WT against Sitophilus zeamais.
| Treatment | LC50 (µL cm-3)a | LC95 (µL cm-3)a | X 2b (df) | Slop±sd |
|---|---|---|---|---|
| Piper coruscans | 7.43 [6.37; 8.59] | 16.07 [13.98; 19.43] | 25.72 (3)c | 0.19±0.03 |
| Piper ottoniaefolium | 3.56 [3.22; 3.85] | 5.95 [5.36; 6.99] | 48.07 (4) | 0.68±0.10 |
| Piper reticulatum | 6.12 [3.45; 8.81] | 28.75 [21.61; 44.35] | 27.98 (3) | 0.16±0.03 |
| Linalol | 2.07 [1.20; 4.03] | 17.45 [14.25; 23.75] | 35.67 (3) | 0.18±0.02 |
| Caryophyllene oxide | - | - | - | - |
| D-WT (chlorpyrifos) | 0.12 [0.11; 0.35] | 5.57 [3.20; 7.25] | 5.89 (4) | 0.14±0.05 |
a LC50 and LC95: insecticide concentrations required to kill 50 or 95% of S. zeamais adults, respectively (CI confidence interval at 95% error probability) (n=5).
bχ 2 Pearson’s chi-square value.
c Degrees of freedom (df).
However, it should be considered that due to the toxicology of the commercial product, it is much more harmful to health than EOs in any case. In addition, it should be taken into account that the insecticidal action can be affected by the volatility of the components of the product (Barra, 2009). Finally, caryophyllene oxide reached a mortality of 13.4±3.77% at 20 µL cm-3 air; these data are not sufficient to be able to estimate mean mortality values, therefore, they were not compared with the commercial product. To date, no insecticidal activity on S. zeamais has been described for the EOs of the Piperaceae included in this research.
Although several studies have demonstrated the biopesticidal action of Piper spp. EOs on S. zeamais, Whitehead and Bowers (2014) tested amide extracts obtained from P. reticulatum on Sibaria englemani, an antagonistic fruit pest. Vásquez-Ocmín et al. (2022) reported antiplasmodial activity of ethanolic extracts of P. coruscans on Plasmodium falciparum. This is the first report of biocidal activity of P. ottoniaefolium EO.
The results obtained in the present study reveal insecticidal activity by fumigant action of EOs of P. coruscans, P. ottoniaefolium and P. reticulatum against S. zeamais. The effectiveness of the EOs may be due to the compounds present in the EOs, mainly monoterpenes (Yildirim et al., 2013). Such is the case of linalool, the substance assayed in this study, which showed 98% mortality at the highest dose after 24 h of exposure. These results coincide with those reported by Kamanula et al. (2017), where linalool obtained 100% mortality against S. zeamais after 48 h of exposure. It should be noted that the commercial insecticide D-WT (chlorpyrifos) was tested up to a concentration of 0.08 μL cm-³, resulting in a mortality of 42.2±0.07% because it was highly insecticidal (Fig. 2). Caryophyllene oxide reported low mortality at all doses analyzed on S. zeamais; this is in agreement with the reports by Ma et al. (2020), where this oxygenated sesquiterpene oxide showed low fumigant toxicity against S. zeamais and M. japonica. However, the antifungal activity of caryophyllene oxide against several species of fungi has been demonstrated (Jassal et al., 2021), as well as its antiproliferative action in the treatment of PC-3 prostate cancer cells (Delgado et al., 2021). In addition, it has shown important anticholinesterase and antioxidant capacities (Karakaya et al., 2020).
CONCLUSIONS
In the present investigation, the chemical composition and insecticidal activity of the EOs of three Piperaceae were studied, showing their potential as effective alternatives for the control of S. zeamais. The results of the chromatographic analysis indicated that the major compounds found in Piper coruscans were caryophyllene oxide (31.75%), and β-selinene (10.29%); in P. ottoniaefolium were the β-bisabolene (14.46%); and α-curcumene (8.36%), and in P. reticulatum were caryophyllene oxide (9.44%), and β-caryophyllene (9.01%). The volatile chemical composition of P. ottoniaefolium EO was described for the first time. The EO of P. ottoniaefolium showed a higher repellency percentage than the other two oils (83.3%) at 2 hours (h) of exposure and 2.5 µg cm-2. In addition, the P. coruscans EO showed the most stable repellency percentage during the test: 2 h (66.7±0.06), 4 h (56.7±0.06) and 6 h (3.3±0.25) at 2.5 µg cm-2. The LC50 of the EOs of P. coruscans, P. ottoniaefolium and P. reticulatum were 7.43, 5.56 and 6.12 in µL cm-3, respectively, and that of the linalool standard was 2.07 µL cm-3. Comparison of means between the EOs and the commercial insecticide product (DW-T) showed statistically significant differences, due to their high toxicity. However, the results of the LC50 and LC95 suggest these essential oils as potential botanical insecticides. Therefore, the essential oils of P. coruscans, P. ottoniaefolium, and P. reticulatum can be a considerable alternative in controlling S. zeamais and creating new biopesticides.















