Introduction
Posterior reversible encephalopathy syndrome (PRES) is a rare complication of various clinical entities. Its incidence is unknown, having been reported in a wide range of ages from 14 to 78 years, with an average age of 44 years and a male/female ratio of 0.8/1.1 Although the prognosis is usually favorable, mortality rates of up to 15% have been reported.2 It is determined by typical clinical-radiological manifestations, usually transient.3 Either in an acute or subacute form, in descending order of frequency it occurs with encephalopathy, seizures, headache, visual disturbances and focal neurological deficit.4 Several pathophysiological theories have been postulated, with two being the most accepted. The first one suggests that the sudden increase in blood pressure exceeds the self-regulation of cerebral blood flow, causing vasodilation and hyperperfusion, with rupture of the blood-brain barrier (BBB) and vasogenic edema.5 Thus, it has been classically associated with eclampsia and hypertensive encephalopathy; however, 20-30% of patients are normotensive, suggesting a second theory of direct endothelial toxicity caused by inflammatory mediators, more correlated to patients with immunosuppressive treatment, renal failure, connective tissue disorders or sepsis.6 It occurs in <1% of patients with systemic lupus erythematosus (SLE), with a higher incidence in young people, with a Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) ≥ 6 and associated comorbidities.7 Magnetic resonance imaging (MRI) of the brain is determinant for the diagnosis, showing vasogenic edema, usually in the posterior cerebral territory, bilateral and symmetrical.8 Since the diagnosis of PRES requires a high clinical and imaging suspicion, with the subsequent establishment of early treatment for a favorable prognosis; the objective of this work is to provide information for the recognition and management of this unusual syndrome associated with SLE.
Description of the case
A 25-year-old mestizo woman, from Quito, Ecuador, with personal pathological antecedents of hypothyroidism and SLE, diagnosed in December 2012, at the age of 21 years. In December 2016, 4 years after the diagnosis of SLE, she presented an exacerbation triggered by acute diarrheal disease; with musculoskeletal (arthritis, myalgias) and mucocutaneous (oral ulcers) manifestations, serositis (right pleural effusion), bicytopenia (anemia, thrombocytopenia), renal involvement (hematuria, proteinuria, acute renal failure, Acute Kidney Injury Network [AKIN] classification III) and arterial hypertension (AHT); all of which conferred her a high SLEDAI (value: 23). The renal biopsy reported focal proliferative lupus glomerulonephritis class II, not corresponding with intense lupus activity; without changes attributable to antiphospholipid syndrome, as well as negativity of these antibodies. Due to the multi-organ involvement, she received pulses of 1 g of methylprednisolone intravenous for 3 days, replacement of blood products, 6 sessions of plasmapheresis, hemodyalisis, amlodipine 10mg/day, atenolol 50mg/day and mycophenolate mofetil 1 g/12 h orally, since she presented gastrointestinal intolerance to higher doses. Three weeks after admission she presented acceptable analytical and clinical improvement with the treatment, and therefore, her hospital discharge was indicated. Twenty-four hours later, the patient re-enters with a convulsive status. In emergencies they initiated airway management, intravenous anticonvulsants with diazepam 10 mg, midazolam 3mg, phenytoin 1g and transferred her to the intensive care unit.
It was requested a cranial tomography, which did not show signs of ischemia or bleeding; a right occipital hypodensity without mass effect was observed, and for this reason the order of exams was broadened to identify its etiology. Metabolic, infectious and pharmacological causes were excluded. Due to AHT difficult to control (up to 190/100 mmHg with mean arterial pressure [MAP] of 130 mmHg), the patient required up to 6 antihypertensive drugs; atenolol 50 mg/12 h, losartan 100 mg/day, amlodipine 10 mg/day, doxazosin 2 mg/6 h via nasogastric tube, and intravenous nitroprusside 50 mg/day and furosemide 20 mg/6 h. Due to severe lupus activity (SLEDAI 21: seizures, hematuria, proteinuria, hypocomplementemia, anti-DNA, thrombocytopenia), she received again treatment with methylprednisolone 1 g/3 days. The electroencephalogram did not show epileptiform activity. It was requested a cerebral angioresonance in which findings consistent with vasculitis or thrombosis of the central nervous system were not found. The MRI of the brain showed typical images of PRES (Fig. 1), whose development would be related to the exacerbated SLE, severe AHT, lupus glomerulonephritis and use of immunosuppressants, so oral nimodipine 60 mg/6 h was added and the trigger factors were controlled. Due to the risk of drug-induced lupus, phenytoin was gradually withdrawn, with progressive increase of levetiracetam up to 1 g/12 h via nasogastric tube. Table 1 details the relevant complementary studies. During the follow-up, she did not present new convulsive events, the renal function remained stationary, the blood pressure values improved (MAP 85-90 mmHg), and the lupus activity decreased (SLEDAI 13: hematuria, proteinuria, hypocomplementemia, anti-DNA, thrombocytopenia). The MRI of the brain of control evidenced involution of the previous lesions (Fig. 2).
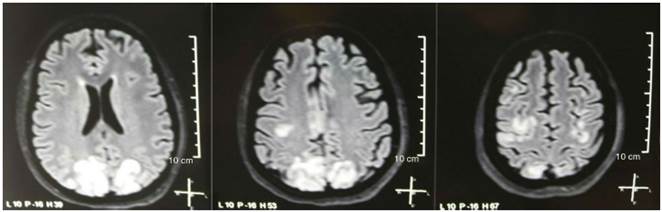
Fig. 1 Simple + diffusion nuclear magnetic resonance imaging of the brain at admission: bilateral, symmetric, hyperintense lesions in FLAIR sequence in white matter of occipital and parietal lobes.
Discussion
Since the first description of the PRES, made in 1996 by Hinchey et al., the knowledge of several aspects of this entity has been broadened. Its original name of reversible posterior leukoencephalopathy syndrome resulted inappropriate, since the imaging changes are not always limited to the cerebral white matter and its clinical manifestations are not always reversible.9 The first 15 cases reported occurred in patients with hypertensive encephalopathy, eclampsia or under immunosuppressive treatment.10 It has also been observed as a complication of other entities such as sepsis, renal failure and connective tissue disorders; therefore, it is currently known that the risk factors that cause endothelial dysfunction are key for the development of PRES.11 The global incidence is not known, but data of retrospective studies indicate that it is more frequent in individuals between 39 and 47 years, generally women, with comorbidities such as hypertensive, renal or autoimmune disorders.12 In patients with SLE, many autoantibodies are directed against the endothelium; producing its activation, expression of adhesion molecules (E-selectin, VCAM-1, ICAM-1) and exposure to proinflammatory cytokines such as IL-1β, TNFα and IL-6, causing disruption of the BBB and appearance of neurological complications.13 It has been reported that in people diagnosed with SLE, the PRES occurs in the context of moderate to severe lupus activity, as well as associated with renal failure and poorly controlled hypertension.14
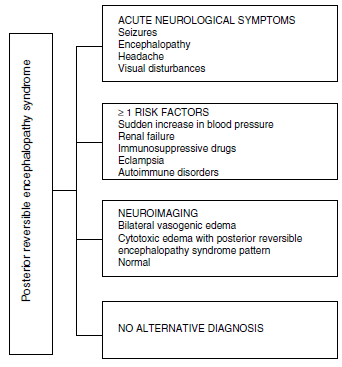
Regarding the clinical manifestations of the PRES, it is characterized by variable degrees of encephalopathy, from confusion to stupor (50-80%), seizures (60-75%), headache (50%) and visual disturbances ranging from blurred vision to cortical blindness (33%); being unusual the focal neurological deficit (10-15%) and the status epileptic (5-15%).15 For the initial assessment of the neurological compromise in these patients, a cranial computed axial tomography (CT) scan is usually requested, which is often normal or can show cortical-subcortical hypodensities, predominantly in posterior brain regions. 16 The MRI of the brain determines the diagnosis, showing vasogenic edema, usually in the white matter of the occipital and parietal lobes (territory of the posterior cerebral circulation), visualized as hyperintense lesions in T2 and FLAIR, bilateral and symmetrical. 17 The preferential involvement of the white matter is due to its structure of myelinated fibers, arterioles and capillaries which confers it greater laxity. Similarly, the vessels of the anterior cerebral circulation, having greater sympathetic innervation, can adequately respond by vasoconstriction to the sudden increase in cerebral blood flow secondary to hypertension; a protective mechanism less developed in the vertebrobasilar system.18 Less frequently, the gray matter and other lobes may be affected. The images with diffusion sequences allow to distinguish between the vasogenic edema, typical of the PRES, and the cytotoxic edema that may occur atypically and can progress to infarction.19 The electroencephalogram does not always correlate with the neurological affectation, but it may reveal encephalopathy by the presence of focal sharp waves. In patients with seizures associated with PRES, the main electroencephalographic alteration is the general slowing in theta/delta frequencies.20 The analysis of the cerebrospinal fluid shows nonspecific changes such as a slight increase in cellularity and proteins, and therefore it is useful when it is convenient to rule out an infection in the central nervous system. 21 In addition to the aforementioned tests, those that are considered necessary for the differential diagnosis, mainly with neurolupus, metabolic and parainfectious encephalopathy, encephalitis, infarction of the posterior cerebral artery and demyelinating disorders must be performed. 22,23 Based on the foregoing, Fig. 3 shows the algorithm proposed by Fugate et al. for the diagnosis of PRES, which aims to identify even atypical cases. 15
In our patient, given the clinical presentation along with the multiple risk factors, imaging findings and exclusion of other etiologies, the diagnosis of PRES was concluded. Symptomatic treatment with anticonvulsant medication and anticerebral edema was instituted in a timely manner, together with the control of the causative factors: severe hypertension, SLE with severe activity, lupus glomerulonephritis, immuno-suppressive drugs; ratifying the diagnosis during the follow-up with the resolution of the clinical and imaging alterations. With respect to the management of the PRES, the blood pressure should be reduced, the seizures should be treated and the trigger should be controlled. The rapid decrease in blood pressure could cause cerebral ischemia, which is why a goal of a mean blood pressure between 105 and 125 mmHg is suggested, without exceeding 25% of this reduction in the first hour. The first-line drugs are calcium channel blockers (nicardipine or nimodipine of choice, that also prevents cerebral vasospasm) or beta-blockers (for example, labetalol). Sodium nitroprusside or hydralazine can be used as second-line drugs. Nitroglycerin should be avoided due to its vasodilator effect, which would increase the cerebral edema 24 The treatment of the seizures is similar to that of other epileptic seizures. Benzodiazepines such as lorazepam or diazepam are used as the first-line therapy. As second-line, phenytoin or valproate, especially in status epilepticus, or phenobarbital. Magnesium sulfate can be used in pregnant women. In refractory seizures, we can give propofol o pentobarbital.25 Drugs that could cause drug-induced lupus, such as hydralazine, methyldopa, captopril, phenytoin, valproate and carbamazepine, should be avoided in patients with SLE. There is controversy about the management of immunosuppressive drugs in the treatment of PRES in patients with SLE.26 After the resolution of the PRES, seizures are infrequent, and therefore, it should be considered to discontinue anticonvulsants as long as there is an adequate control of the risk factors.27 With a timely and adequate treatment, the majority of patients with PRES evolve satisfactorily with remission of the symptoms and imaging lesions in a few days or weeks, although complications, specially hemorrhagic, have been observed in 9-33% of cases, so this case highlights the importance of its recognition and management, which is often a challenge.28
Conclusions
The diagnosis of PRES requires a high clinical and imaging suspicion. Timely treatment with control of the symptoms and the underlying cause ratifies the diagnosis during follow-up, with the resolution of clinical and imaging alterations; otherwise, it may cause neurological sequelae or death.











 text in
text in