Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Earth Sciences Research Journal
Print version ISSN 1794-6190
Earth Sci. Res. J. vol.17 no.2 Bogotá July/Dec. 2013
HYDROGEOLOGY
A multivariate statistical approaches on physicochemical characteristics of ground water in and around Nagapattinam district, Cauvery deltaic region of Tamil Nadu, India
S. Venkatramanan1, 2, S. Y. Chung1*, T. Ramkumar2, G. Gnanachandrasamy2, S. Vasudevan2
1 Institute of Environmental Geosciences, Department of Earth & Environmental Sciences, Pukyong National University, 599-1 Daeyeon-dong Nam-gu, Busan 608-737, South Korea
2 Department of Earth Sciences, Annamalai University, Annamalai Nagar 608-502, Tamil Nadu, India
*Corresponding author email: chungsy@pknu.ac.kr, venkatramanansenapathi@gmail.com
Manuscript received: 03/12/2012
Accepted for publication: 01/11/2013
ABSTRACT
Ground water samples collected at different locations in and around the Nagapattinam district were analyzed for their physicochemical characteristics. The ground water samples were collected from fifty two dug and deep wells during the monsoon and summer seasons in June and December, 2011. The present investigation is focused on the determination of physico-chemical parameters such as pH, EC, TDS, Ca, Mg, Na, K, HCO3, SO4 and Cl. Ground water suitability for drinking, domestic and agricultural purposes was examined by using WHO standards. Correlation, factor and cluster analyses were applied to classify the ground water qualities and to categorize the geochemical processes controlling ground water geochemistry. Factor analysis indicates that seawater intrusion and agriculture runoff are dominant factors controlling the hydrogeochemistry of ground water in the study area. Cluster analysis was helpful for the classification on the basis of contamination characteristics of ground water quality. This study also elucidates that multivariate statistical analyses can be used to improve the understanding of ground water status and assessment of ground water quality.
Key words: Ground water quality, multivariate statistical analyses, ground water suitability, correlation, factor and cluster analyses.
RESUMEN
En este estudio se analizan las características fisicoquímicas de muestras de aguas subterráneas tomadas en diferentes locaciones en y alrededor del distrito de Nagapattinam. Las muestras se recolectaron en 52 pozos cavados y perforaciones profundas durante las subestaciones del monzón y el verano, en los meses de junio y diciembre (2011). La presente investigación está enfocada en la determinación de parametros fisicoquímicos como pH, EC, TDS, Ca, Mg, Na, K, HCO3, SO4 y Cl. Se examinó la pertinencia de estas aguas para consumo y para irrigación a la luz de los estándares de la Organización Mundial de la Salud. Se aplicaron análisis de correlación, factores y cúmulos para clasificar las muestras y categorizar los procesos geoquímicos que controlan las aguas subterráneas. El análisis de factores indica que la intrusión del agua marina y los vertidos de la agricultura son los principales agentes que intervienen la hidroquímica de las aguas subterráneas en el área de estudio. El análisis de cúmulos ayudó a la clasificación de las bases de contaminación características de las aguas subterráneas. Este estudio, además, dilucida que los estudios estadísticos multivariantes pueden ser utilizados para mejorar el conocimiento y la evaluación de la calidad de las aguas subterráneas.
Palabras clave: Calidad de aguas subterráneas, análisis estadístico, incursión de agua marina, Nagapattinam, costa este de la India.
Introduction
One of the most severe problems concerning ground water quality is the rapid salinization of water resources. The increase in ground water salinity, particularly in coastal areas, may be due to the inflow of natural saline water, such as sea water intrusion, dissolution of soluble salts in the unsaturated zone, and saline water naturally derived from adjacent aquifers (Vengosh and Rosenthal, 1994, Vengosh et al., 1999, Maslia and Prowell, 1990, Jones et al., 1999, Rosenthal et al., 1992, Glynn and Plummer 2005, Cloutier et al., 2008, Brinda and Elango, 2011). Anthropogenic contamination is another major cause of salinization and water-quality degradation. Ground water irrigation causes salts recycling and accumulation in aquifers. The salts are accumulated in the soil and flushed through the unsaturated zone to the aquifer. Moreover, irrigation with wastewater, which is generally more saline than regional ground water, increases the salinization rate of shallow ground water. This problem is more conspicuous in arid and semi-arid zones, where potable water is replaced by wastewater for irrigation in order to save the depleting water resources. Ground water salinization and degradation is usually observed through the sampling of pumping wells. In these wells, the screens are located in the deeper parts of the aquifer, far below the water table. In the light of this fact, one of the management strategies for ground water resources especially in the coastal belt is to ensure that ground water extraction does not lead to the intrusion of saline seawater into the aquifers. However, due to the relatively low elevations in the area and the lack of strict regulation, ground water salinization continues to be a problem in some of the coastal areas. On the other hand, this resource is the only source of water for agricultural activities in view of the erratic nature of rainfalls in recent times. The high salinity of ground water and the heavy reliance on ground water for irrigation necessitate the overall assessment of ground water quality for irrigation purposes. (Hussein, 2004, Ramkumar et al. 2011, Anithamary et al. 2012, Venkatramanan et al. 2012, Gnanachandrasamy et al. 2012). Thus, the suitability of ground water for irrigation and drinking purposes thus had to determined based on the presence of major ions in the ground water of this coastal region. This study proposes using multivariate statistical analysis to identify a correlation between fresh and saline water incursion.
Description of the study area
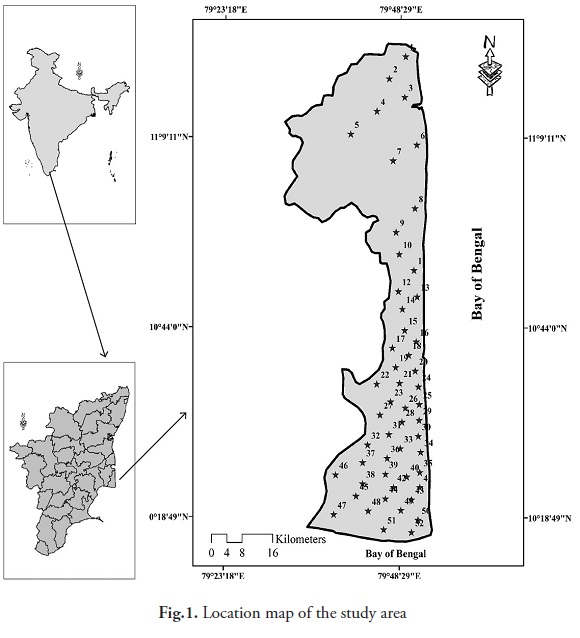
The study area is located in east coast of India, and its stretches between Coleroon in the north and Kodikarai in the south (Fig. 1). The district has a coastline stretching of about 190 km. The Nagapattinam district mainly comprises of the Quaternary sediments, which increases towards the southern part of Coleroon River. These sediments have been delineated as alluvial plain deposits of the Cauvery River and its tributaries, narrow fluvio-marine deltaic plain deposits and marine coastal plain deposits (east coast formation). The fluvial deposits comprise of flood plain, flood basin, point bar, channel bar and palaeo channels with admixtures of sand, silt and clay. The deltaic plain includes palaeo tidal flats with clays, and sands and sand ridges or gray brown sand. The marine coastal plains include beach, tidal flats, salt marsh, mangrove swamps, and deposits of sand and clay. The Cretaceous Formations of the coastal track of the Cauvery Basin consist of faunal, rich marine sedimentary rocks, such as limestones, sandstones, clays and sandy beds, etc. The mouth of the river comprises of alluvial deposits including clays and silts.
Ground water occurs in these formations and is extracted by filter point wells, tube wells, shallow boreholes and infiltration wells, especially from the sandy aquifers. The Pliocene and Quaternary shallow aquifers consists of sand, gravel and clay. The aquifer is more clayey towards the east and south eastern part of the district, except the coastal stretch of beach sands. The depth to shallow aquifers varies between 3 m and 35 m, whereas the depth to deep aquifers ranges from 80 m to 100 m. The average annual rainfall in the monsoon season is about 451.48 mm, whereas during that of summer, rainfall averages is about 43.12 mm.
Method of study
Fifty two ground water samples were collected from different water sources such as dug and bore wells in June and December, 2011, using high-density polythene containers. The water samples were analyzed for major physico-chemical parameters adopting standard procedures (APHA 1995). pH was measured by a pH pen (eco Tester pH) and electrical conductivity (EC), total dissolved solids (TDS), Ca, Na, Mg, K, HCO3, Cl, and SO4 were measured using a multi-parameter ion analyzer (Hanna, HI83099). All concentrations are expressed in milligrams per litre (mg/l), except pH and EC. Multivariate statistical analyses were performed to find out determine the relationship between ground water parameters using Statistica Software (ver.8). The results were evaluated in accordance with the norms prescribed under 'WHO' (World Health Organization, 1993) (Table 2).
Results and Discussion
Summary of ground water analysis
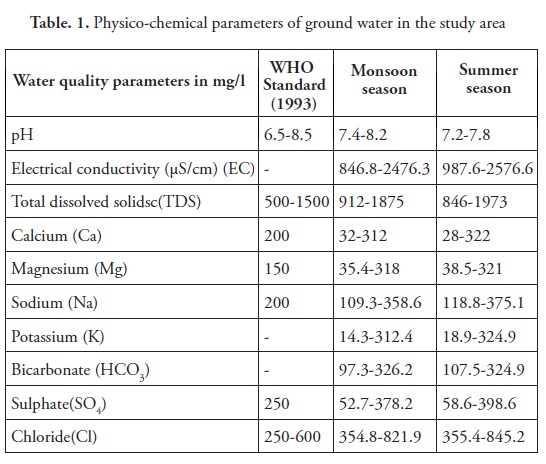
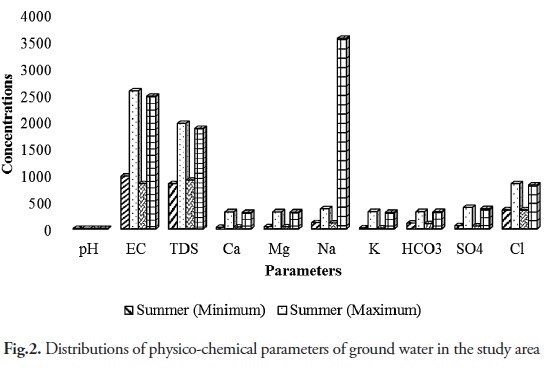
The water quality analysis of different ground water samples were carried out to determine different physicochemical parameters such as for pH, EC, TDS, Ca, Mg, Na, K, HCO3, SO4, and Cl; and the results are presented in Table 1 and Figure 2. pH value of ground water samples between 7.4-8.2 and 7.2-7.8 during the monsoon and summer seasons, respectively. Ground water is a little alkaline, but the quality is within the limiting value of the drinking water standard. The general increase of pH in a sedimentary terrain is related to weathering of plagioclase feldspar in sediments. This is aided by dissolved atmospheric carbon dioxide resulting in the release of sodium and calcium, which progressively increases pH and alkalinity of the ground water. EC varied to 846.8-2476.3 and 987.6-2576.6 µS/cm, and TDS changed to 912-1875 mg/l and 846-1973 mg/l during monsoon and summer seasons, respectively. EC and TDS exceed WHO (1993) permissible limits. The summer season recorded high levels of EC and TDS compared to the monsoon season. The reasons are the intrusion of residual solids into the aquifer, the movement of water through sediments containing higher soluble mineral matter, and the influx of industrial and municipal wastes with incursion of seawater. The calcium and magnesium concentrations are generally derived from the leaching of limestone, dolomites, gypsum and anhydrites (Garrels 1976, Ramkumar et al. 2010, Anithamary et al. 2012). Calcium and magnesium ranged between 32-312 mg/l and 28-322 mg/l, respectively during the monsoon sub season, and 35.4-318 mg/l and 38.5-321 mg/l, respectively during the summer season. Most of the ground water samples exceed WHO (1993) permissible limits in Ca and Mg. The increased concentration in Ca and Mg in ground water samples is due to the leaching of limestone, dolomites, gypsum and anhydrites (Aiuppa et al., 2003, Banaszuk et al., 2005, Samsudin et al., 2008). The ionic concentration ranged between 109.3-358.6 mg/l and 118.8-375.1 mg/l for sodium, and 354.8-821.9 mg/l and between 355.4-845.2 mg/l for chloride, respectively. Thus, sodium might have been derived from the dissolution of evaporated minerals and its subsequent mixing with ground water. The higher Na and Cl concentration ground water in the coastal region is attributed to the influence of seawater on the coastal aquifer, which was highly visible during the summer due to a decline in water table (Einsiedl 2012, Kouzana et al. 2010, Yidana, and Yidana, 2009). Ionic concentrations were 14.3-312.4 mg/l for potassium, and 18.9-324.9 mg/l for bicarbonate, and 97.3-326.2 mg/l for sulphate during the monsoon season; and 107.5-324.9 mg/l for potassium, 52.7-378.2 mg/l for bicarbonate, and 58.6-398.6 mg/l for sulphate during the summer season, respectively. The large portion of potassium is due to the intrusion of saline water as well as the fertilizers of agricultural activity.
In this study, it was observed that Na, K, Ca, and Mg were higher in summer than in monsoon season. Na and Mg were lower, but K and Ca were higher during monsoon. It indicates that the seasonal variation of cation concentrations was very conspicuous in this environment. The relative abundance of sodium and magnesium during the summer revealed the influence of monsoon rainwater on the ground water. It led to an increase in these elements from irrigation runoff, thereby increasing their concentration in the ground water during summer season. The weathering of plagioclase feldspar and the nature of dissolved atmospheric carbon dioxide releases sodium and calcium, which progressively increases the sodium and calcium concentrations. Chloride and sulfate were dominated during summer season, but chloride showed more variations than sulfate during two seasons. It is resulted from agricultural activity and seawater incursion in the shallow aquifer system.
Correlation between variables
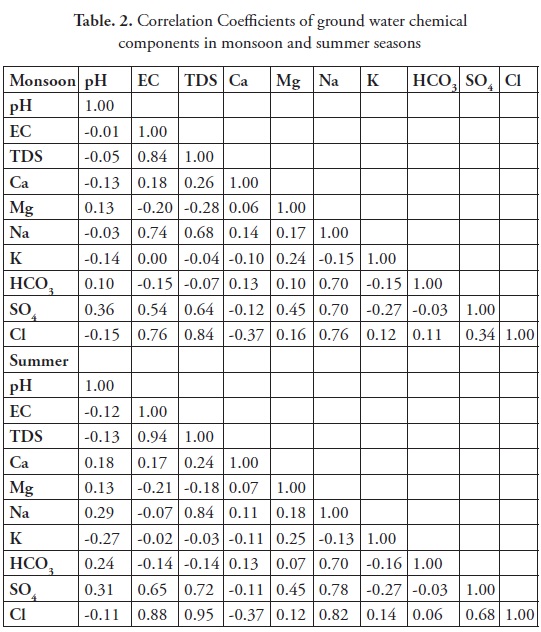
The correlations between major cations and anions were carried out using Pearson's correlation. A correlation analysis is a bivariate method applied to describe the degree of relation between hydrochemical parameters. Associated the variable representing with correlation coefficient is r, and the multiple correlations, which is the percentage of variance in the dependent variable, is explained collectively by all of the independent variables. The results of the correlation analysis are considered in the subsequent interpretation section. A high correlation coefficient (near 1 or -1) means implies a good strong relationship between the two variables, and a correlation coefficient around of approximately zero means implies that there is no relationship between them two variables, at a significance level of, 0.05. More precisely, it can be said that parameters showing r > 0.7 are considered to be strongly correlated, whereas when r has a value between 0.5 and 0.7, a moderate correlation is shown is said to exist (Kim et al. 2002; Helena et al. 2000, Shammas and Jacks, 2007). Positive values of r indicate a positive relationship, while negative values indicate an inverse relationship. The correlation coefficients of the studied parameters are shown in Table 2. In the present study, EC and TDS are strongly correlated with Na, Cl and SO4 during the monsoon, and similar type of result was observed during summer. They thus indicate seawater incursion and leaching of secondary salts. The lower concentration of calcium compared with sodium and chloride is a result of the cation exchange process that occurs naturally when seawater is introduced into the freshwater aquifer system (Appelo and Postma 2005, Zeng and Rasmussen, 2005, Venkatramana et al., 2009). Furthermore, during the summer season, sodium is positively correlated with Cl, EC, and TDS, indicating seawater incursion due to continuous pumping activities in the study area. The Ca shows significant correlation with EC and TDS and good correlation was noted in HCO3. The Na was strongly correlated with Cl, clearly indicating that the seawater incursion plays a vital role in this present study (Ramkumar et al., 2011 and Anithamary et al 2012).
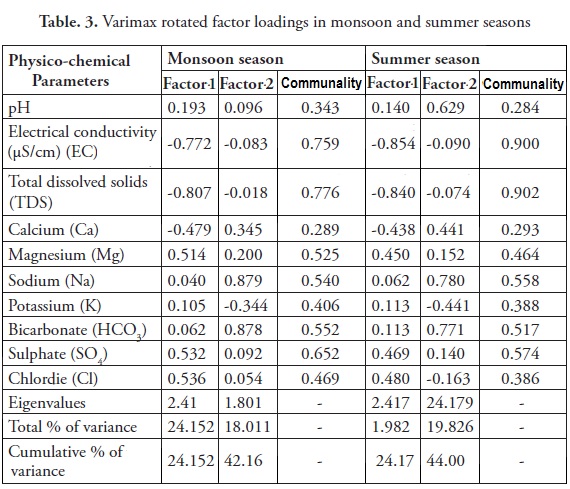
Varimax rotation factor analysis
Factor analysis is a frequent common statistical technique employed in hydrochemical studies (Subbarao et al., 1996, Adami et al., 1997, Davis, 1986). The main goals of factor analysis are: (1) to reduce the number of 'variables'and (2) to detect structure in the relationships between 'variables'(analytical parameters) and/or 'cases'(sampling points in our study). The mathematical base of factor analysis and a review of the chemical aspects can be found in Mermet et al, (1998). Currently, the calculations of factor analysis calculations and their graphical results performed using by Davis (1986).
Consequently during the summer season, the first factor accounts for 24.17% whereas the second factor accounts for 44.00% (Table 3). Factors 1 and 2 show a good strong relationship with EC, TDS, HCO3, Na, Cl and Mg, which is indicative of seawater incursion and agricultural runoff incursions in the study area. The concentration of Na and Cl can be ascribed to the incursion of seawater into the aquifer systems. Furthermore, during the monsoon season, the first factor accounts for 24.15% and second factor accounts for 42.16%. The factor analyses performed in this study has clearly indicated that seawater incursion into the coastal aquifer as is the primary source of ground water pollution, the similar observations was made by Venkatramanan et al. (2012).
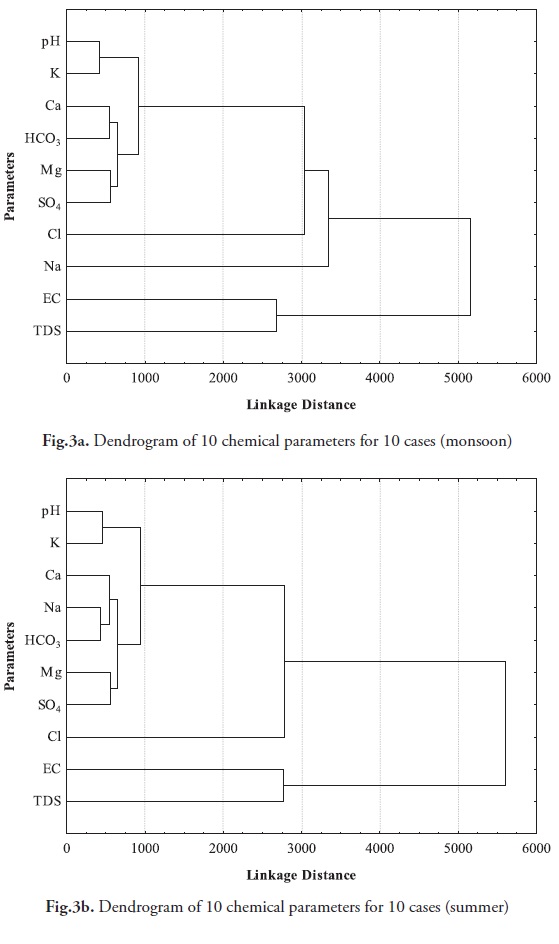
Cluster analysis
Cluster analysis is used for grouping the cases based on the similarity of the responses to several variables. On the basis of the connecting distances between parameters and their positions on the dendrogram, distinctive clusters of the variables were defined for each of three locations along the basin. Though this procedure is subjective, the distinction between clusters in this analysis is quite clear from the dendrogram. Cluster analysis is a useful method for combining ground water wells into homogenous groups according to their water quality. In this study, three types of clustering nature was observed during the monsoon and summer seasons. The first clusters are pH, K, Ca, Na, HCO3 and SO4, whereas the second cluster is Cl accompanied by pH, K, Ca, Na, HCO3 and SO4. The third cluster is accompanied by EC and TDS. These variables would be found in natural mineralization and anthropogenic processes. The non-homogenous nature of the aquifer led to different hydrogeochemical facies for ground water such as Na and Cl. A close relationship between Na and Cl, may be originated from sea water incursion into coastal aquifers (Figs. 3a, b).
Conclusions
The problem of ground water in Nagapattinan district coastal region is rather complicated, and is derived from excessive exploitation of the aquifer systems. The study area depends totally on the water derived from the aquifer for its irrigation use. The overpumping of fresh water caused the deterioration of the water quality by the upwelling of deep saline water. Fifty two ground water samples were collected near the coastal village of the Nagapattinam district during monsoon and summer seasons, and physico-chemical characteristics of the samples were studied to determine the quality and the suitability of the ground water for various purposes as well as the characteristics of ground water quality. The maximum concentrations were observed near the coastal area because of sea water incursion. In addition, irrigation return flow also plays a major role in controlling ground water quality in the Nagapattinam district. A dendrogram of the 10 cases and 10 variables are plotted and grouped into three main clusters. The values of correlation coefficients and their significance levels contributed to the selection of proper treatments to minimize the contamination of the ground water in the study area. The factor analysis reveals that the ground water from Nagapattinam area has been greatly influenced by lithologic and environmental events in the area. A continuous monitoring program of the water quality will help to avoid further deterioration of the ground water quality in this coastal region.
Acknowledgements
The authors are grateful to two anonymous referees for their constructive comments and suggestions which led to significant improvements to the manuscript. We also thank for the valuable suggestions given by Carlos Alberto Vargas Jimenez, Editor, which greatly helped in the final presentation of the paper. This research was supported by a grant (code 13AWMP-B066761-01) from AWMP Program funded by the Ministry of Land, Infrastructure and Transport of Korean government and Department of Science and Technology (Project No: DST/WAR-W/WSI/02/2010), New Delhi, India.
References
Adami, G., Barbieri, P., Picciotto, A. and Reisenhofer, E. (1997). Principal factor analysis as applied in environmental chemistry - the study of eutrophication in a shallow lake, Toxicological of Environmental Chemistry, 61, 99–108. [ Links ]
Anitha Mary, I., Ramkumar, T. and Venkatramanan, S. (2012). Application of Statistical Analysis for the Hydrogeochemistry of Saline Groundwater in Kodiakarai, Tamilnadu, India, Journal of Coastal Research, 28, 89–98. [ Links ]
Aiuppa, A., Bellomo, S., Brusca, L., Alessandro, W. and Federico, C. (2003). Natural and anthropogenic factors affecting groundwater quality of an active volcano (Mt. Etna, Italy) Applied Geochemistry, 18, 863–882. [ Links ]
APHA. (1995). Standard methods for the examination of water and waste water, 19th edn. NewYork, USA. [ Links ]
Appelo, C. A. J. and Postma, D. (2005). Geochemistry, Groundwater and Pollution, 2nd edition. Rotterdam: Balkema. [ Links ]
Banaszuk, P., Wysocka-Czubaszek. and A., Kondratiuk. (2005). Spatial and temporal patterns of groundwater chemistry in the river riparian zone Agriculture, Ecosystems and Environment, 107, 167–179. [ Links ]
Brindha, R. and Elango, L. (2011). Hydrochemical characteristics of groundwater for domestic and irrigation purposes in Madhuranthakam, Tamil Nadu, India, Earth Sciences Research Journal, 15, 101-108. [ Links ]
Cloutier, V., Lefebvre, R., Therrien, R. and Savard, M. M. (2008). Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. Journal of Hydrology, 353, 294–313. [ Links ]
Desimone, L. A., Howes, B. L. and Barlow, P. M. (1997). Mass-balance analysis of reactive transport and cation exchange in a plume of wastewater-contaminated groundwater, Journal of Hydrology, 203, 228–249. [ Links ]
Einsied, F. (2012). Sea-water/groundwater interactions along a small catchment of the European Atlantic coast, Applied Geochemistry, 27, 73–80. [ Links ]
Engosh, A. and Keren, R. (1996). Chemical modifications of groundwater contaminated by recharge of sewage effluent, Journal of Contamination Hydrology, 23, 347–360. [ Links ]
Garrels, R. M. (1976). A survey of low temperature water mineral relations in interpretation of environmental isotope and hydrogeochemical data in groundwater hydrology: Vienna, International Atomic Energy Agency, 22, 65–84. [ Links ]
Glen, E. P., Cohen, M. J., Morrison, J. I., Valdes-Casillas, C. and Fitzsimmons, K. (1999). Science and policy dilemmas in the management of agricultural waste waters: the case of the Salton Sea, Ca, USA, Environmental Science Policy, 2, 413–423. [ Links ]
Glynn, P. D. and Plummer, L. N. (2005). Geochemistry and the understanding of groundwater systems, Hydrogeology Journal, 13, 263–287. [ Links ]
Gnanachandrasamy, G., Ramkumar, T., Venkatramanan, S., Anithamary, I., Vasudevan, S. (2012) GIS based hydrogeochemical characteristics of groundwater quality in Nagapattinam district, Tamil Nadu, India. Carpathian Journal of Earth and Environmental Sciences, 7, 205–210. [ Links ]
Hao, X. and Change, C. (2002). Does long-term heavy cattle manure application increase salinity of a clay loam soil in semi-arid southern Alberta? Agriculture Ecosystem Environment, 18, 1–16. [ Links ]
Hern, J. and Feltz, H. R. (1998). Effects of irrigation on the environment of selected areas of the Western United States and implications to world population growth and food production, Journal of Environmental Management, 52, 353–360. [ Links ]
Helena, B., Pardo, R., Vega, M., Barrado, E., Fernandez, J. M. and Fernandez, L. (2000). Temporal evolution of groundwater composition in alluvial aquifer (Pisuerga River, Spain) by principal component analysis, Water Research, 49, 359-372. [ Links ]
Hussein, M. T. (2004). Hydrochemical evaluation of groundwater in the Blue Nile Basin, eastern Sudan, using conventional and multivariate techniques, Hydrogeology Journal, 12, 144–158. [ Links ]
Jones, B. F., Vengosh, A., Rosenthal, E. and Yechieli, Y. (1999). Geochemical Investigations, in: Bear, J., Cheng, A. H. D., Sorek, S., Ouazar, D., Herrera, I. (Eds.), Seawater Intrusion in Coastal Aquifers-Concepts, Methods and Practices. Kluwer, Dordrecht, 51–72. [ Links ]
Kanfi, Y., Ronen, D. and Magaritz, M. (1983). Nitrate trends in the Coastal Plain aquifer of Israel, Journal of Hydrology, 66, 331–341. [ Links ]
Kouzana, L., Benassi, R., Mammoua, A. B. and Mennoubi, S. (2010). Geophysical and hydrochemical study of the seawater intrusion in Mediterranean semi arid zones. Case of the Korba coastal aquifer (Cap-Bon, Tunisia), Journal of African Earth Sciences, 58, 242–254. [ Links ]
Kim, R. H., Yum, B. W. and Chang, H. W. (2002). Hydrogeochemical and isotopic characteristics for salinization of a shallow groundwater in coastal area, Youngkwang, Korea, Proc. 17th Salt Water Intrusion Meeting, Delft, The Netherlands, 227–237. [ Links ]
Maslia, M. L. and Prowell, D. C. (1990). Effect of faults on fluid flow and chloride contamination in a carbonate aquifer system, Journal of Hydrology, 115, 1–49. [ Links ]
Mermet, J. M., Otto, M. and Widmer, H. M. (1998). Analytical Chemistry, Wiley, New York. [ Links ]
Mitchel, J. P., Shennan, C., Singer, M. J., Peters, D. W., Miller, R. O., Prichard, T., Grattan, S. R., Rhoades, J. D., May, D. M. and Munk, D. S. (2000). Impacts of gypsum and winter cover crops on soil physical properties and crop productivity when irrigated with saline water, Agriculture Water Management, 45, 55–71. [ Links ]
Ramkumar, T., Venkatramanan, S., Anithamary, I. and Syed Ibrahim, S. (2011). Evaluation of hydrogeochemical parameters and quality assessment of the groundwater in Kottur blocks, district, Tamilnadu, India, Arabian Journal of Geosciences, doi:10.1007/s12517-011-0327-2. [ Links ]
Ramkumar, T., Venkatramanan, S., Anitha Mary, I., Tamilselvi, M. and Ramesh, G. (2010). Hydrogeochemical quality of groundwater in Vedaraniyam Town, TamilNadu, India, Research Journal of Environmental and Earth Sciences, 2, 44-48. [ Links ]
Ronen, D., Kanfi, Y. and Magaritz, M. (1983). Sources of nitrates in groundwater of the Coastal Plain of Israel—evolution of ideas, Water Research, 17, 1499–1503. [ Links ]
Ronen, D. and Magaritz, M. (1985). High concentration of solutes at the upper part of the saturated zone (water table) of a deep aquifer under sewage irrigation land, Journal of Hydrology, 80, 311–323. [ Links ]
Rosenthal, E., Vinokurov, A., Ronen, D., Magaritz, M. and Moshkovitz, S. (1992). Anthropogenically induced salinization of groundwater: A case study from the Coastal Plain aquifer of Israel, Journal of Contamination Hydrology, 11, 149–171. [ Links ]
Samsudin, A. R., Haryono, A., Hamzah, U. and Rafek, A. G. (2008). Salinity mapping of coastal groundwater aquifers using hydrogeochemical methods: a case study from North Kelantan, Malaysia, Environmental Geology, 55, 1737–1743. [ Links ]
Sreekanth, J. and Datta, B. (2010). Multi-objective management of saltwater intrusion in coastal aquifers using genetic programming and modular neural network based surrogate models, Journal of Hydrology, 393, 245–256. [ Links ]
Shammas, M. I. and Jacks, G. (2007). Seawater intrusion in the Salalah plain aquifer. Oman, Environmental Geology, 53, 575– 587. [ Links ]
Subbarao, C., Subbarao, N. V. and Chandu, S. N. (1996). Characterization of groundwater contamination using factor analyses, Environmental Geology, 28, 175–180. [ Links ]
Vengosh, E. and Rosenthal, E. (1994). Saline groundwater in Israel; its bearing on the water crisis in the country, Journal of Hydrology, 156, 389–430. [ Links ]
Vengosh, A., Spivack, A. J., Artzi, Y. and Ayalon, A. (1999). Geochemical and boron, strontium and oxygen isotopic constraints on the origin of the salinity in groundwater from the Mediterranean coast of Israel, Water Resource Research, 35, 1877–1877. [ Links ]
Venkatramanan, S., Ramkumar, T., Vasudevan, S., Ramesh, G., Anitha Mary, I. and Vijayakumar, V. (2009). Assessment of hydrogeochemistry of groundwater in Muthupet coastal region, Tamilnadu, India, International Journal of Applied Environmental Sciences, 4, 169–176. [ Links ]
Venkatramanan, S., Ramkumar, T. and Anithamary, I. (2012). A Statistical approach on hydrogeochemistry of groundwater in muthupet coastal region, Tamilnadu, India, Carpathian Journal of Earth and Environmental Sciences, 7, 47 – 54. [ Links ]
Yidana, S. M. and Yidana, A. (2009). Assessing groundwater quality using water quality index and multivariate statistical analysis-the Voltaian basin, Ghana, Environmental Earth Science, 42, 132– 145. [ Links ]
Zeng, X. and Rasmussen, C. D. (2005). Multivariate statistical characterization of water quality in Lake Lanier, Georgia, USA, Journal of Environmental Quality, 34, 1980–1991. [ Links ]
WHO. (1993). Fluorine and Fluorides, Environmental Health Criteria, 36. World Health Organization, Geneva. [ Links ]