Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.34 no.2 Bogotá Apr./June 2019
https://doi.org/10.22516/25007440.396
Case report
Menetrier disease: Case report with video
1Médico internista y gastroenterólogo, Hospital Universitario Nacional de Colombia, Universidad Nacional de Colombia, Unidad de gastroenterología y ecoendoscopia (UGEC). Bogotá D. C., Colombia
2Médico internista y gastroenterólogo, Hospital Universitario Nacional de Colombia, Unidad de gastroenterología y ecoendoscopia (UGEC). Bogotá D. C., Colombia
Menetrier disease (also known as giant hypertrophic gastritis or hypoproteinemic hypertrophic gastropathy) is a rare entity characterized by protein losing enteropathy, hypochlorhydria and thickening of the mucosal folds of the fundus and the gastric corpus. Its constellation of classic symptoms includes nausea, vomiting, abdominal pain and peripheral edema, and it is associated with increased risk of gastric cancer. Nevertheless, its pathophysiology is not yet fully understood and clinical and endoscopic diagnosis can be difficult to establish. This article describes a clinical case and provides a brief review of the literature.
Keywords: Menetrier disease; mucosal hypertrophy; hypoalbuminemia
La enfermedad de Ménétrier, también conocida como gastritis hipertrófica gigante o gastropatía hipertrófica hipoproteinémica, es una entidad poco frecuente, caracterizada por una gastroenteropatía perdedora de proteínas, hipoclorhidria y engrosamiento de los pliegues mucosos del fondo y el cuerpo gástrico; es causante de un grupo clásico de síntomas que incluyen náuseas, vómitos, dolor abdominal y edema periférico; se asocia con un mayor riesgo de cáncer gástrico, sin embargo, su fisiopatología aún no está del todo esclarecida y su diagnóstico, clínico y endoscópico, puede llegar a ser difícil de establecer, por lo que se describe un caso clínico y se presenta una revisión sucinta de la literatura.
Palabras clave: Enfermedad de Ménétrier; hipertrofia mucosa; hipoalbuminemia
Introduction
The French pathologist Pierre Menetrier (1859-1935) first described the disease that bears his name in the Archives de Physiologie Normale et Pathologique in 1888. Menetrier described seven individuals who exhibited two different macroscopic patterns of gastric hypertrophy: polypoid adenomas and sheet-like polyadenomas. He likened the patterns of the thickened gastric mucosa to cerebral convolutions. 1,2 The Office of Rare Diseases of the National Institute of Health of the United States of America considers Menetrier disease to be rare, which means that its prevalence is less than 1 in 200,000 individuals. It is sometimes known by other names, including giant hypertrophic gastritis and hypoproteinemic hypertrophic gastropathy. 2 Since there are no pathognomonic characteristics for diagnosing Menetrier’s disease, diagnosis is based on clinical and pathological characteristics. This, together with its rarity, poses a diagnostic and therapeutic challenge.
Clinical case
The patient was a 19-year-old man who began to suffer from abdominal pain and distention at 12 years of age during late childhood and early adolescence. His weight and height were both low for his age. He had been treated by different specialties until 2016 when he came to our service for upper digestive tract endoscopy as part of an evaluation requested by the attending physician. Thick gastric folds were found in the fundus and corpus with clearly decreasing distensibility (Video 1). From the clinical point of view, asthenia and dyspepsia were the predominant symptoms. During physical examination the patient was pale and had edema grade II in his lower limbs.
Video 1. Endocopy of Ménétrier’s disease. Thickened proximal gastric folds affected by edema can be seen. https://youtu.be/sQNxWFhjeq0
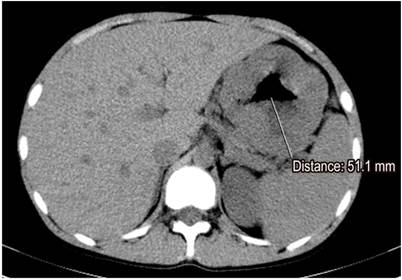
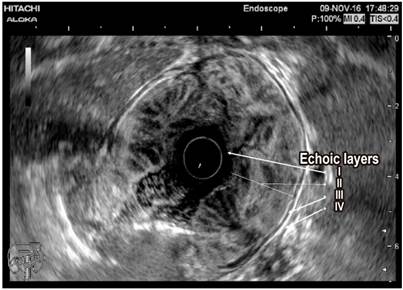
Paraclinical tests including a complete blood count, albumin, nitrogen and urine analysis were requested. The patient was found to have normocytic, normochromic, heterogeneous anemia. His hemoglobin level was 11.2 g/dL, his serum albumin level was 2.8 g/dL, and his creatinine level was 0.8 mg/dL (normal). The urine analysis did not find proteinuria. Given the clinical, paraclinical and endoscopic findings, computed tomography (CT) of the abdomen was performed. It found thickened gastric walls with diffuse, marked and symmetric gastric folds without evidence of nodular lesions. The maximum thickness was 53 mm (Figure 1). Findings from gastric endoscopic ultrasonography (EUS) were similar to those described of the upper digestive tract endoscopy, but thickening of the gastric wall dependent on the first and second echoic layers (mucosa and muscular mucosa, respectively) was found. Anechoic spaces were found in the second echoic layer respecting the third and fourth echoic layers (submucosa and muscularis propria, respectively) (Figure 2).
The histology report from biopsies taken in the upper digestive endoscopy showed hyperplastic gastritis with “Menetrier’s disease pattern, and the patient was negative for Helicobacter pylori (Operative Link on Gastritis Assessment [OLGA]: 0). A follow-up in July 2017 found the patient’s symptoms due of abdominal pain and distention were worsening and that there was associated vomiting, nausea and anasarca. Surgical management was decided upon.
Discussion
Menetrier’s disease is most often found in men between the ages of 30 and 60 years although cases have also been reported in childhood. Clinically, patients present abdominal pain, nausea, vomiting and edema of the peripheral tissues (imbalance of osmotic pressure due to the selective filtration of proteins through the gastric mucosa). 3. This disease tends to be progressive, although its pathophysiology is still unknown. Transgenic mice models overexpress transforming growth factor alpha (TGF-α) in the stomach and undergo changes that resemble those found in Menetrier’s disease. In addition, the receptor for epidermal growth factor (EGF) in foveolar mucus cells is overstimulated by TGF-α, its ligand, which causes excess mucus secretion and malabsorption of nutrients.
From the clinical point of view, onset is usually insidious and progressively includes characteristics that are associated with increased risks of gastric cancer. Although the magnitude of this risk is not entirely clear, various authors place it between 0% and 10%. 3,4 Variants with abrupt onsets have also been described. These have been reported most frequently in relation to spontaneous remission related to treatment of associated cytomegalovirus (CMV) infection or H. pylori infections. Some authors have also described associations with autoimmune diseases such as inflammatory bowel disease, sclerosing cholangitis and ankylosing spondylitis which suggests that there is an immunological component which has not yet been fully elucidated. 2,5
Endoscopically, the folds of the gastric mucosa are markedly thick especially in the fundus and the corpus rather than in the antrum. Gastric pH is high due to the loss of parietal cells, and there is copious production of thick mucus secondary to foveolar hyperplasia that occurs most commonly in the mucosa. This causes mucosal thickness to increase by one cm or more (in our clinical case it reached 5 cm). This is a necessary condition for diagnosis. 5
Histological alterations include reduced numbers of parietal cells and main cells and atrophied oxyntic glands. Deep glands may be cystically dilated and predominantly chronic inflammatory cells with dispersed eosinophils infiltrate the lamina propria in variable amounts. Smooth muscle hyperplasia and edema are associated with decreased numbers of fundic glands which are replaced by mucous glands (pseudopyloric metaplasia). This totally abnormal mucosal architecture generates loss of protein which is frequently increased by superficial ulcers. 4,5,6
Differential diagnosis revolves around other entities that thicken gastric folds. These include lymphocytic gastritis, polyposis syndromes, hyperplastic polyps, plastic lymphadenitis and lymphoma (Table 1). EUS is a useful tool for differential diagnosis since it can exclude a thickening of vascular origin in cases where biopsies may cause significant bleeding. Consequently, it is recommended that EUS precede any decision to take biopsies in cases of thickening of gastric folds. Thickening originating in the second echoic layer supports a diagnosis of Menetrier’s disease (Figure 2). 6,7
Table 1 Differential Diagnosis
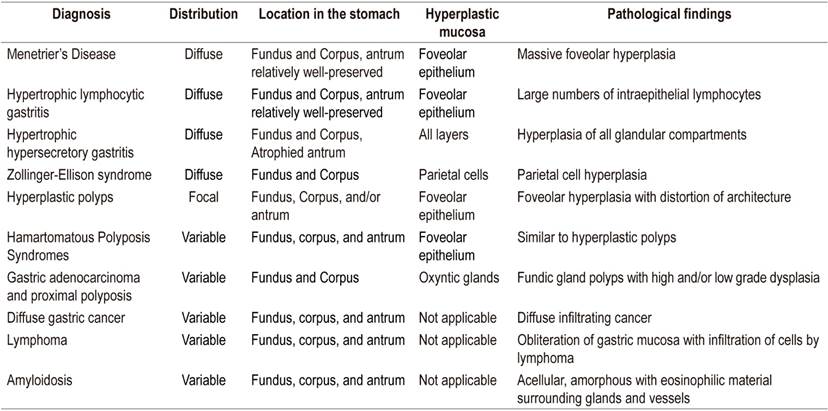
Taken from: Silva PH et al. Rev Assoc Med Bras (1992). 2016; 62 (6): 485-9.
Treatment is usually surgical, and partial or total gastrectomy is currently considered the treatment of choice. Nevertheless, several drug therapies have been proposed. They include weekly administration of cetuximab which has improved patients’ quality of life. Despite this, some patients followed up for 40 months required long-term gastrectomy, so the use of cetuximab has only been recommended as the first line for management of Menetrier’s disease in cases of relapses after gastrectomies. 6 Other drugs including famotidine and cimetidine have shown favorable results including reports of decreased symptoms. In the case of cimetidine, decreased protein loss has also been reported. Steroids and antibiotics have also been used but with conflicting results. It should be noted that, given the low prevalence of this disease, none of these treatments have had clinical trials with the required methodological rigor, so all reports are now considered anecdotal experiences. 8
Conclusion
Menetrier’s disease is recognized as a rare disease, so its diagnosis is difficult. Nevertheless, it is of crucial importance given the risk of associated malignancy. Based on available evidence, the currently recommended treatment is predominantly surgical, although there are other treatments that can be implemented in specific clinical situations such as relapse.
Referencias
1. Ménétrier P. Des polyadenomes gastriques et leur rapport avecle cancer de l›estomac. Arch Physiol Norm Pathol. 1888;1:236-62. [ Links ]
2. Rich A, Toro TZ, Tanksley J, Fiske WH, Lind CD, Ayers GD, et al. Distinguishing Ménétrier’s disease from its mimics. Gut. 2010;59(12):1617-24. https://doi.org/10.1136/gut.2010.220061 [ Links ]
3. Coffey RJ Jr, Tanksley J. Pierre Ménétrier and his disease. Trans Am Clin Climatol Assoc. 2012;123:126-33. [ Links ]
4. Huh WJ, Coffey RJ, Washington MK. Ménétrier’s Disease: Its Mimickers and Pathogenesis. J Pathol Transl Med. 2016;50(1):10-6. https://doi.org/10.4132/jptm.2015.09.15 [ Links ]
5. Patel M, Mottershead M. Disease recurrence following cetuximab completion and declining a gastrectomy: what next to manage Ménétriers disease? BMJ Case Rep. 2014;2014. pii: bcr2014204954. https://doi.org/10.1136/bcr-2014-204954 [ Links ]
6. Fiske WH, Tanksley J, Nam KT, Goldenring JR, Slebos RJ, Liebler DC, et al. Efficacy of cetuximab in the treatment of Menetrier’s disease. Sci Transl Med. 2009;1(8):8ra18. https://doi.org/10.1126/scitranslmed.3000320 [ Links ]
7. Azer M, Sultan A, Zalata K, Abd El-Haleem I, Hassan A, El-Ebeidy G. A case of Menetrier’s disease without Helicobacter pylori or hypoalbuminemia. Int J Surg Case Rep. 2015;17:58-60. https://doi.org/10.1016/j.ijscr.2015.10.025 [ Links ]
8. Silva PH, Rigo P, Batista RP, Toma RK, Oliveira LA, Suzuki L. Ménétrier’s disease associated with gastric adenocarcinoma in a child - imaging aspect. Rev Assoc Med Bras (1992) . 2016;62(6):485-9. https://doi.org/10.1590/1806-9282.62.06.485 [ Links ]
Received: January 30, 2018; Accepted: March 18, 2018











 text in
text in