Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista MVZ Córdoba
Print version ISSN 0122-0268On-line version ISSN 1909-0544
Rev.MVZ Cordoba vol.24 no.2 Córdoba May/Aug. 2019
https://doi.org/10.21897/rmvz.1309
Research article
Superovulatory response in Brahman donor cows using follicular ablation before superovulation protocols
1Corporación Colombiana de Investigación Agropecuaria (AGROSAVIA), Km 14 vía Bogotá - Mosquera, Colombia.
2Universidad Federal de Pará, Laboratorio de fertilización In Vitro, Instituto de Ciencias Biológicas, Belém, Pará, Brasil.
Objective.
The objective of this study was to determine the effect of follicular ablation at the beginning of a superovulation protocol (SOP) on the superovulatory response of Brahman donor cows.
Materials and methods.
Twenty Brahman cows were used, randomly distributed in two groups: control group (G1, n = 10), synchronization of the follicular growth wave was performed by the combination of estrogens (2.5 mg, estradiol benzoate) and progestagens (1 gr intravaginal implant); four days after starting the SOP with porcine follicle stimulating hormone (FSHp); and the ablation group (G2, n = 10), follicular ablation was performed and one day after, the SOP treatment with FSHp was initiated. In both groups, embryo collection was performed seven days after the first artificial insemination.
Results.
The G2 had a higher proportion of quality 1 embryos (p<0.01) compared to G1 (68.60% vs. 31.22%), while animals of G1 group had a higher proportion of quality 2 embryos (43.04% vs. 18.60%, p<0.01). For the total of structures collected and the total of transferable embryos, no significant differences were observed (p>0.05).
Conclusions.
Follicular ablation increased the percentage of quality 1 embryos, suggesting that the implementation of this technique, as a strategy to synchronize the beginning of a new wave of follicular growth when using SOP, improve embryo quality in Brahman donor cows.
Keywords: Bovine; embryo; follicular aspiration; reproduction (Fuente: MeSH)
Objetivo.
Determinar el efecto de la ablación folicular en el inicio de un protocolo de superovulación (SPO) sobre la respuesta superovulatoria en vacas donantes de raza Brahman.
Materiales y métodos.
Se utilizaron 20 vacas de raza Brahman, las cuales fueron distribuídas aleatoriamente en dos grupos: Grupo control (G1; n = 10), la sincronización de la onda de crecimiento folicular fue realizada mediante la combinación de estrógenos (2.5 mg, Benzoato de Estradiol) y progestágenos (1 gr, implante intravaginal); cuatro días después se inició el protocolo de SPO con la hormona folículoestimulante porcina (FSHp); y grupo ablación (G2; n = 10), se realizó la ablación folicular y un día después se inició el tratamiento de SPO con FSHp . En los dos grupos la colecta de los embriones se realizó siete días después de la primera inseminación artificial.
Resultados.
El G2 presentó una mayor proporción de embriones de calidad 1 (p<0.01) en comparación con el G1 (68.60%, 31.22%), mientras que los animales del grupo G1 presentaron una mayor proporción de embriones de calidad 2 (43.04%, 18.60%, p<0.01). Para las variables total de estructuras colectadas, y total de embriones transferibles, no se observaron diferencias significativas (p>0.05).
Conclusiones.
La ablación folicular aumentó el porcentaje de embriones de calidad 1, sugiriendo que la implementación de esta técnica, como estrategia para sincronizar el inicio de una nueva onda de crecimiento folicular en tratamientos de SPO, mejora la calidad de los embriones producidos en vacas donadoras Brahman.
Palabras clave: Aspiración folicular; bovinos; embrión; reproducción (Fuente: MeSH)
INTRODUCTION
The use of superovulation protocol (SOP) technique for the production and embryo transfer (ET), has been one of the most widely disseminated biotechnologies in breeding programs applied to the bovine species 1. However, the rates of embryo production have remained static over time (6.2 viable embryos collected by donor) 2. The variability in the response to SOP treatments on embryo donors remains the biggest problem in commercial programs of ET 3. This variability could be related to extrinsic factors, to the own treatment of the SOP treatment, or mainly, intrinsic factors, the follicular dynamics particularities of each donor, such as the size of the follicular population or the number of follicular waves, among others 4.
Variations in follicular dynamics are an important factor in SOP. The SOP treatments that do not start at the time of the appearance of a follicular wave have a significant reduction in the superovulatory response 5. In addition, the number of corpus luteum, the collected structures, and the transferable embryos decrease in cows submitted to SOP treatments that present a dominant follicle (DF) at the beginning of treatment, this fact is related to the DF capacity to induce the atresia process in the subordinate follicles, affecting the development capacity 6. In this way, the implementation of strategies aiming to manipulate follicular dynamics and synchronize the onset of the follicular wave with the start of the SOP treatments is determinant to reduce the variability of the SOP protocols 7.
The manipulation of follicular dynamics to avoid the presence of dominant follicles is traditionally done by hormonal treatments with an association of estrogens and progestagens 8. Other treatments consist of the application of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), human chorionic gonadotropin (hCG) or its analogues, in any of these cases, the appearance of the new follicular wave will occur between 24 and 48 hours after the treatment, depending on the hormone used, and the synchronization will only be effective in animals that ovulate in response to the treatment 6,9. It has also been shown that synchronization of a new follicular wave appearance can be mechanically induced by follicular ablation, aspirating all the present follicles, ≥ 5 mm in diameter, or eliminating the dominant follicle, in either case, the appearance of the new follicular wave occurs approximately between 24 and 48 hours later 10,11.
Although some differences in the reproductive physiology characteristics between the Bos Taurus and Bos indicus have been reported 12,13, the causes of the variation on the superovulatory response in Bos indicus animals, subspecies to which belongs the Brahman breed, correspond to the same described for of Bos Taurus origin donors 14,15. In the other hand, reports describing the effects of the implementation of follicular ablation on SOP protocols and that have been evaluated on Brahman cows are limited in the literature. Taking together, it is important to generate alternatives that contribute in improving the SOP technique efficiency and the in vivo embryo production, and mainly that promotes standardization of the results, the present study aimed to determine the effect of follicular ablation at the beginning of an SOP on the superovulatory response of Brahman donor cows.
MATERIAL AND METHODS
Study site. The present study was conducted at the Oklahoma farm, located in the municipality of San Alberto, Cesar (Latitude: 7.7627, Longitude: -73.3931 7° 45'46'' North, 73° 23' 35'' West), with an altitude of 125 m, average temperature of 28.1°C and an annual rainfall regimen of 2223 mm 16.
Animals. Twenty non-lactating multiparous Brahman cows were used, with an average weight of 510±50 kg, average age of 7±2 years, average body condition of 3.5±1 [scale of 1 to 5 as described by Pereira et al. 17 ], and the absence of visible alterations in the reproductive tract. The animals were also reared at the same environmental, nutritional conditions (water, mineralized salt, ad libitum grazing), management, selection and administration of the treatments. The 20 cows were randomly distributed into two groups (control group, n = 10 and ablation group, n = 10).
Experimental groups and superovulation protocols.
Prior to the beginning of the SOP, in order to confirm the ovarian cyclicity (presence of a corpus luteum), a gynecological evaluation was performed by transrectal ultrasonography, using a 5 to 8 MHz multifrequency transducer (Mindray DP 2200 vet; Mindray, Shenzhen, China).
Control Group (G1). The time of insertion of an intravaginal device impregnated with 1.0 g of progesterone (P4) in association with an injection of estradiol benzoate (EB) equivalent to 2.5 mg, intramuscular (im) was considered as day zero (D0). On the fourth day of the protocol, the SOP treatment was initiated by applications of the porcine follicle stimulating hormone (FSHp). For this purpose, a total dose of 200 mg of FSHp was divided into eight decreasing doses, distributed in two daily applications with 12-hours interval, in am/pm scheme, over a period of four days (40, 40, 30, 30, 20, 20, 10 and 10 mg, im respectively). Concomitant with the fifth and sixth application of FSHp (day 6 of the protocol), the animals received two doses of prostaglandin F2a (PGF2a), each one corresponding to 25 mg, Dinoprost tromethamine im, additionally, the intravaginal device was withdrawn together with the sixth application of FSHp, and finally, 36 hours after the withdrawal of the P4 device (day 8 of the protocol), 0.021 mg, buserelin acetate im (GnRH) were administered. The schematic design of the experimental groups can be observed in figure 1.
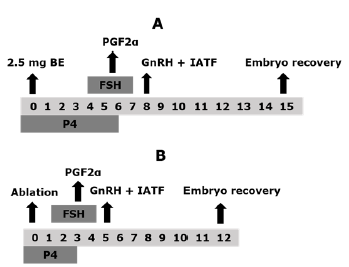
Figure 1 Schematic design of the experimental groups. Control group (G1, n = 10, A) and ablation group (G2, n = 10, B). Estradiol benzoate (EB), Prostaglandin F2a (PGF2a), Gonadotropin releasing hormone (GnRH), fixed time artificial insemination (FTAI), Follicle stimulating hormone (FSH), Progesterone (P4).
Ablation Group (G2). The animals in this group were initially subjected to follicular ablation, which was considered the D0 of the protocol. The ablation consisted in the mechanical suction of the ovarian follicles ≥ 5 mm, using an ultrasound device (Mindray DP 2200 vet; Mindray, Shenzhen, China), coupled to a micro-convex multifrequency transducer from 5 to 8 MHz, which fitted to a guidewire for transvaginal ultrasonography used in follicular aspiration procedures (WTA, Cravinhos, Brazil). Through the guide, the aspiration system was placed, which consisted of a disposable 20 G x 23/4" needle, which was communicated, by a suction line, to a 50 mL plastic tube (Falcon, Becton Dickinso Labware, New York, USA). The vacuum pressure was generated with the help of a 50 mL disposable syringe. The follicular ablation procedure was performed according to described by Acosta et al 18, in the following way: initially, the upper space area of the first coccygeal vertebrae was disinfected with 70% alcohol solution, and in this region 5 mL of 2% lidocaine hydrochloride was injected.
The vulva and perineal region were washed and disinfected with iodine solution and 70% alcohol. Subsequently, the follicular aspiration guide was introduced to the bottom of the vaginal sac and after fixing the ovary against the transducer by manipulation through the rectum, the needle was moved until perforating the vaginal wall and entering the ovarian stroma. Twenty four hours after the follicular ablation (D1 of the protocol) the SOP treatment was started, following the doses, products and application scheme described for G1. Briefly, for G2 the intravaginal progesterone implant was placed on D0, immediately after the ablation was performed, the treatment of SOP with FSHp was administered during days one to four of the protocol, on day three (D3) the two doses of PGF2a were applied (scheme am/pm) and with to the second dose of PGF2a (D3, pm) the implant was removed, finally, the application of the GnRH analog was performed on day five (D5) (Figure 1).
For the two groups, a total of three artificial inseminations (AI) were carried out in each animal, the first being performed at the time of the GnRH injection (D8 for G1 and D5 for G2), and the remaining two doses carried out with intervals of 12 hours from the first AI. In each AI a semen dose of 40 million sperm was used in 0.5 cc straws, which was thawed at 36°C for 40 seconds, and with the use of the insemination gun, the seminal content was deposited in the body of the uterus. For all cases, straws corresponding to the same Brahman bull and to the same freezing lot were used. Finally, the collection of the embryos was performed seven days after the first AI (day 15 of the protocol for the G1 and day 12 for the G2, Figure 1).
Embryo collection. Prior to the procedure, the donor perineal area was washed, disinfected, and epidural anesthesia was induced by the injection of 5 ml of 2% lidocaine. The recovery of the embryos was carried out by a gravity uterine lavage closed-circuit, using phosphate-buffered saline (PBS) supplemented with 2% bovine serum albumin (BSA) and antibiotics (Bioniche animal health Inc, Canada). The search and classification of the embryos was performed through a stereoscopic microscope (Nikon SMZ645, Nikon, Japan) equipped with a thermal stage at 37°C. For the maintenance of the structures during the evaluation period, a holding media formulated with PBS and supplemented with 20% BSA and antibiotics (Bioniche animal health).
The embryo quality was evaluated according to the morphological characteristics of the structures, following the parameters established by the International Society of Embryo Technologies (IETS) (19), as follows: Quality 1 embryos: (excellent or good) development corresponding to the day of the collection, colorful blastomeres, size, and uniform texture, spherical shape and intact zona pellucida. At least 85% of the cell mass material is intact. Quality 2 embryos: (fair) presence of irregularities in the cell mass, in the color or size of the cells, presence of vesicles and loose blastomeres. At least 50% of the cell mass is intact. Quality 3 embryos: (poor) presence of severe defects in cell mass, loose blastomeres, degenerated cells of different size and numerous vesicles. 25% of the cell mass remains intact. Quality 4 embryos: (dead and degenerate) disorganized blastomeres, degenerated and loose, with vesicular appearance, granular and delayed growth in relation to the developmental stage.
Statistical analyses. The statistical analysis was performed using the statistical software SAS 9.4 (Institute Inc. Cary, NC, USA) using the Student's T test for the total structures collected and the total of transferable embryos per animal. The Shapiro-Wilk test was used to determine the normal distribution of the data, when the parameters were not adjusted to normal, a logarithmic transformation was used for a better homogeneity of the residuals distribution and back transformed for data presentation. The Chi-square test was used to analyze the proportion of degenerated embryos, quality 1, quality 2 and unfertilized oocytes in relation to the total collected structures. Statistical significance was declared at p<0.05.
Ethical aspects. Considering the guidelines and ethical standards for carrying out research procedures with animals, all the experimental procedures carried out in this study were approved by the Ethics Committee for Research of the Pedagogical and Technological University of Colombia (UPTC) through the Minutes No. 05 of March 20, 2018.
RESULTS
No significant differences were observed between groups for the total of structures collected per animal (p=0.502) and total of transferable embryos per animal (p=0.201, Table 1).
Table 1 Superovulatory response of Brahman donor cows to hormonal superovulation protocols (SOP). Control group and ablation group (follicular ablation prior to SPO treatment).
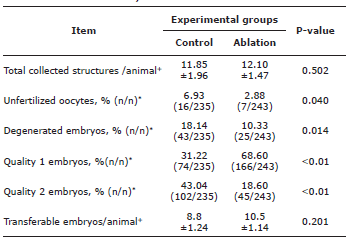
* Values (n/n) correspond to the number of each variable, in relation to the total of structures. +Values represent average ± standard deviation. Statistical significance was declared as p<0.05.
However, donor cows subjected to follicular ablation produced a higher proportion of quality 1 embryos compared to the animals of the control group (p<0.01). Although, a higher percentage of quality 2 embryos (p=0.01) was obtained from animals in control group (Table 1). Likewise, a higher percentage of degenerated embryos and unfertilized oocytes were observed in the control group (p>0.05, Table 1).
DISCUSSION
In this study, two synchronization methods for a new follicular wave emergence were evaluated for SOP and ET purposes, and can be designated, in a general way, as a physical and pharmacological method. However, regardless of the method, the rate of recovery of transferable embryos/animal obtained was high (8.8±1.24 and 10.5±1.14). According to the IETS 20 in South America, 7.5 transferable/collected embryos are produced on average and 6.7 transferable/collected embryos are produced in the world, lower values than those observed in the present study.
Certain factors that were considered as selection criteria for the donors that participated in the experiment, such as ovarian cyclicity (corpus luteum presence), adequate body condition, age and productive status (no lactating), and also the subspecies to which they belong. This could have influenced the result. Particularly, bovine females of Bos indicus origin have high circulating concentrations of insulin-like growth factor (IGF) compared to Bos taurus animals, this characteristic has been related to a high follicular population of this subspecies 21, consequently, when working with Brahman cows, a considerable number of follicles are available, presenting greater possibilities of an adequate response to SOP treatments 13,22.
When comparing the two methods, it was observed that the use of follicular ablation increased the percentage of quality 1 embryos. Many studies have shown that the absence of a dominant follicle in the beginning of follicular growth stimulation with exogenous FSH is one of the most impacting factors in the superovulatory response, not only regarding the number of ovulations, but also in the quality of the recovered structures 4,23.
The use of combined steroids, such as P4 and E2, to synchronize the beginning of a follicular growth wave is based on the suppressive effect of P4 on the pituitary release of gonadotropins, mainly on LH, together with follicular atresia, caused by E2, through the transient effect of FSH concentrations suppression 7. Once the
E2 concentrations decrease, as a consequence of the metabolization of this molecule, FSH increase, allowing the beginning of a new wave of follicular growth 3. However, it has been observed that sometimes these treatments fail in the suppression of dominant follicles, allowing them to persist even after treatment, generating an inhibitory effect on the development of the other follicles 24. Likewise, functional dominant follicles present before and at the beginning of treatment with P4 and E2 may have exerted different degrees of atresia on the remaining follicular population 24. In any cases, the degree of atresia suffered by each follicle, reduce in a variable form the oocytes quality, affecting the embryos final quality, generated after the fertilization process 25. On the other hand, the mechanical removal of larger follicles (≥5 mm) could be a more effective method to prevent the presence of functionally dominant follicles, resulting in a more homogeneous group of small renewed follicles that grow synchronously by effect of exogenous FSH, consequently, oocytes with a higher degree of competence can be ovulated at the end of treatment 23,27.
The factors associated with the effect that follicular cells have on oocytes competence are related to the activity exerted by the different substances (growth factors, microRNAs, amino acids, fatty acids, carbohydrates, among others) and that are produced in a paracrine way or released through secretory vesicles in bidirectional sense, aiming to coordinate the proper development of growing oocytes 26. In this sense, the viability of the follicular cells is an essential requirement to ensure the correct communication between these types of cells, and thus also the later development competence of the oocyte.
Regarding the variables, infertile oocytes and degenerated embryos, it was found a greater proportion of these structures in the control group. These results are related to the same factors discussed above, which have influenced the quality of the oocytes and, therefore, in the proportion of fertilized oocytes and in the quality of the harvested embryos 25. However, for these variables, Baracaldo et al 10 and Surjus et al 27, did not found differences when using follicular ablation. The number of unfertilized oocytes and the normal development potential of the embryos also depend on the capacity of the sperm used on AI process 28. The presence of alterations in the sperm cells, mainly the relationships with the presence of DNA fragmentation spots, have been related to fails in the subsequent cellular divisions of the blastomeres, which correlates with fragmentation degree, as in the embryonic development, or even, issues on fertilization process 29. Differences in the quality of the seminal material used for AI in the different studies, could explain the divergence on the results.
In the present study no differences were detected between groups for total collected structures and total transferable embryos per animal. Similarly, Baracaldo et al 10 and Surjus et al 27 reported that although the number of harvested embryos in the response to ablation, there was no difference in the number of transferable embryos when this technique was compared with the conventional pharmacological protocol. The number of collective structures, and within these, the total produced embryos, correspond to variables directly influenced by the size of the follicular population present in the animals and by the ovulatory response of these structures to the hormonal treatments, being independent characteristics of oocyte quality, when it is not highly compromised 30.
In conclusion, the use of follicular ablation increased the proportion of quality 1 embryos, suggesting that the application of this technique could replace the traditional treatment with steroids at the beginning of a superovulation protocol, this may result in a better embryonic survival rates after cryopreservation in commercial programs of ET. More studies are needed to determine the effects of follicular ablation on the superovulatory response. The inclusion of molecular analysis evaluating the oocyte and embryo quality could corroborate to understand the mechanisms that are altered by the ablation process at the beginning of the superovulation protocols.
Acknowledgment
To Oklahoma farm where the procedures were carried out. To Dra. Carolina Bespalhok Jacometo for the document translation.
REFERENCES
1. Baruselli PS, Ferreira RM, Sales JNS, Gimenes LU, Sá Filho MF, Martins CM, et al. Timed embryo transfer programs for management of donor and recipient cattle. Theriogenology. 2011; 76(9):1583-1593. DOI: https://doi.org/10.1016/j.theriogenology.2011.06.006 [ Links ]
2. Blondin P. Status of embryo production in the world. Anim Reprod. 2015; 12(3) 356-358. DOI: https://doi.org/10.1080/09613218.2015.993536 [ Links ]
3. Bó GA, Carballo D, Tríbulo A, Tríbulo H, Tríbulo R, Rogan D, et al. New approaches to superovulation in the cow. Reprod Fertil Dev. 2009; 22(1):106-112. DOI: https://doi.org/10.1071/RD09226 [ Links ]
4. Kohram H and Poorhamdollah M. Relationships between the ovarian status and superovulatory responses in dairy cattle. Anim Reprod Sci. 2012; 131(3-4):123-128. DOI: https://doi.org/10.1016/j.anireprosci.2012.03.004 [ Links ]
5. Hasler JF. Forty years of embryo transfer in cattle: A review focusing on the journal Theriogenology, the growth of the industry in North America, and personal reminisces. Theriogenology. 2014; 88(1):152-169. https://doi.org/10.1016/j.theriogenology.2013.09.010 [ Links ]
6. Mapletoft RJ, Bó GA. The evolution of improved and simplified superovulation protocols in cattle. Reprod Fertil Dev. 2011; 24(1):278-283. DOI: https://doi.org/10.1071/RD11919 [ Links ]
7. Mapletoft RJ, Bó GA. Innovative strategies for superovulation in cattle. Anim Reprod. 2013; 10(3):174-179. http://www.cbra.org.br/portal/downloads/publicacoes/animalreproduction/issues/download/v10n3/p174-179%20(AR613).pdf [ Links ]
8. Bó GA, Mapletoft RJ. Historical perspectives and recent research on superovulation in cattle. Theriogenology. 2014; 81(1):38-48. DOI: https://doi.org/10.1016/j.theriogenology.2013.09.020 [ Links ]
9. Aslan S, Arslanbas D, Beindorff N, Bollwein H. Effects of Induction of Ovulation with GnRH or hCG on Follicular and Luteal Blood Flow in Holstein-Friesian Heifers. Reprod Dom Anim. 2011; 46(5):781-786. DOI: https://doi.org/10.1111/j.1439-0531.2010.01741.x [ Links ]
10. Baracaldo MI, Martinez M, Adams GP, Mapletoft RJ. Superovulatory response following transvaginal follicle ablation in cattle. Theriogenology. 2000; 53(6):1239-1250. DOI: https://doi.org/10.1016/ S0093-691X(00)00268-5 [ Links ]
11. Honparkhe M, Gandotra VK, Matharoo JS, Ghuman SPS, Dadarwal D, Jaswant Singh. Synchronization of follicular wave emergence following ultrasound-guided transvaginal follicle ablation or estradiol-17p administration in water buffalo (Bubalus bubalis). Anim Reprod Sci. 2014; 146(1-2):5-14. DOI: https://doi.org/10.1016/j.anireprosci.2014.02.006 [ Links ]
12. Sartori R, Bastos M, Baruselli PS, Gimenes L, Ereno R, Barros CM. Physiological differences and implications to reproductive management of Bos taurus and Bos indicus cattle in a tropical environment. Soc Reprod Fertil Sup. 2010; (67):357-375. DOI: https://www.ncbi.nlm.nih.gov/pubmed/21755684 [ Links ]
13. Silva-Santos KC, Siloto LS, Santos GMG, Morotti F, Marcantonio TN, Seneda MM. Comparison of Antral and Preantral Ovarian Follicle Populations between Bos indicus and Bos indicus taurus Cows with High or Low Antral Follicles Counts. Reprod Dom Anim. 2013; 49(1):48-51. DOI: https://doi.org/10.1111/rda.12222 [ Links ]
14. Soares JG, Martins CM, Carvalho NAT, Nicacio AC, Abreu-Silva AL, Campos EP, et al. Timing of insemination using sex-sorted sperm in embryo production with Bos indicus and Bos Taurus superovulated donors. Anim Reprod Sci. 2011; 127(3-4):148-153. DOI: https://doi.org/10.1016/j.anireprosci.2011.08.003 [ Links ]
15 Sartori R and Barros CM. Reproductive cycles in Bos indicus cattle. Anim Reprod Sci. 2011; 124(3-4)1244-250. DOI: https://doi.org/10.1016/j.anireprosci.2011.02.006 [ Links ]
16. Climate-data. Historical average temperatura. Clima San Alberto [en línea]. Climate-data; 2018; https://es.climate-data.org/america-del-sur/colombia/cesar/san-alberto-34400/ [ Links ]
17. Pereira LL, Ferrreira AP, Vale WG, Sequire LR, Neves KAL, Morini AC, et al. Effect of body condition score and reuse of progesterone-releasing intravaginal devices on conception rate following timed artificial insemination in nelore cows. Reprod Dom Anim. 2018; 53(3):624-628. DOI: https://doi.org/10.1111/rda.13150 [ Links ]
18. Acosta DAV, Rivelli MI, Skenandore C, Zhou Z, Keisler DH, Luchini D, et al. Effects of rumen-protected methionine and choline supplementation on steroidogenic potential of the first postpartum dominant follicle and expression of immune mediators in Holstein cows. Theriogenology. 2017; 96:1-9. DOI: https://doi.org/10.1016/j.theriogenology.2017.03.022 [ Links ]
19. Robertson I and Nelson RE. Certification and identification of embryos; In: Stringfellow D and Givens MD, editor. Fourth edition of manual of the international embryo transfer society (IETS). Champaign, Illinois 61822 USA. 2010; 95-108. [ Links ]
20. Perry G. 2016 statistics of embryo collection and transfer in domestic farm animals. International embryo technology society (IETS) data retrieval committee. [En linea]. 2016. https://www.iets.org/pdf/comm_data/IETS_Data_Retrieval_Report_2016_v2.pdf [ Links ]
21. Satrapa RA, Razza EM, Castilho ACS, Simões RAL, Silva CF, Nabhan T, et al. Differential Expression of IGF Family Members in Heat-Stressed Embryos Produced In Vitro from OPU-Derived Oocytes of Nelore (Bos indicus) and Holstein (Bos taurus) Cows. Reprod Dom Anim. 2013; 48(6):1043-1048. DOI: https://doi.org/10.1111/rda.12211 [ Links ]
22. Batista EOS, Macedo GG, Sala RV, Ortolan MDDV, Sá Filho MF, Del Valle TA, el at. Plasma Antimullerian Hormone as a Predictor of Ovarian Antral Follicular Population in Bos indicus (Nelore) and Bos Taurus (Holstein) Heifers. Reprod Dom Anim. 2014; 49(3):448-452. DOI: https://doi.org/10.1111/rda.12304 [ Links ]
23. Amiridis GS, Tsiligianni T, Vainas E. Follicle Ablation Improves the Ovarian Response and the Number of Collected Embryos in Superovulated Cows During the Early Stages of Lactation. Reprod Dom Anim. 2006; 41(5):402-407. DOI: https://doi.org/10.1111/j.1439-0531.2006.00684.x [ Links ]
24. Ginther JO and Hoffman MM. Intraovarian effect of dominant follicle and corpus luteum on number of follicles during a follicular wave in heifers. Theriogenology. 2014; 82(1):169-175. DOI: https://doi.org/10.1016/j.theriogenology.2014.03.013 [ Links ]
25. Baruselli PS, Sa Filho MF, Ferreira RM, Sales JNS, Gimenes LU, Vieira LM, Mendanha MF, Bo GA. Manipulation of follicle development to ensure optimal oocyte quality and conception rates in cattle. Reprod Dom Anim. 2012; 47(4): 134-141. DOI: https://doi.org/10.1111/j.1439-0531.2012.02067.x [ Links ]
26. Nuttinck F. Oocyte related factors impacting on embryo quality: relevance for in vitro embryo production. Anim Reprod. 2018; 15(3):271-277. DOI: https://doi.org/10.21451/1984-3143-AR2018-0077 [ Links ]
27. Surjus RS, Prata AB, Borsato M, Mattos F, Martins da Silveira MC, Mourão GB, et al. In vivo embryo production in cows superovulated 1 or 2 days after ovum pick-up. Reprod Fertil Dev. 2013; 26(4):527-532. DOI: https://doi.org/10.1071/RD12398 [ Links ]
28. Samardzija M, Getz I, Lojkic M, Valpotic H, Djuricic D. Optimization of Sperm for In vitro Production of Bovine Embryos. SOJ Vet Sci. 2015; 1(2):1-7. DOI: http://dx.doi.org/10.15226/2381-2907/1/2/00107 [ Links ]
29. Simon L, Murphy MB, Shamsi L, Liu B, Emery KI, Aston J, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014; 29(11):2402-2412. DOI: https://doi.org/10.1093/humrep/deu228 [ Links ]
30. Silva SK, Santos GM, Koetz JC, Morotti F, Siloto LS, Marcantonio TN, et al. Antral Follicle Populations and Embryo Production - In Vitro and In Vivo - of Bos indicus-taurus Donors from Weaning to Yearling Ages. Reprod Dom Anim. 2014; 49(2):228-232. DOI: https://doi.org/10.1111/rda.12255 [ Links ]
How to cite (Vancouver) Pérez-Sandoval L, Dubeibe-Marin D, Chavez-Rodriguez A, Garcia-Jimenez J, Velasco-Acosta D. Superovulatory response in Brahman donor cows using follicular ablation before superovulation protocols. Rev MVZ Cordoba. 2019; 24(2):7203-7208. DOI: https://doi.org/10.21897/rmvz.1309
Creative Commons Attribution 4.0 International License This article is distributed under the terms of the (https://creativecommons.org/licenses/by-sa/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source. 
Received: July 01, 2018; Accepted: November 01, 2018; Accepted: April 19, 2019











 text in
text in 


