INTRODUCTION
Coral reefs constitute one of the most valuable, productive, and biodiverse ecosystems on the planet, offering various ecosystem services to the coastal population; they are a source of great economic value for traditional fishing and tourism (Birkeland, 1997; Buddemeier et al., 2004; Burke et al., 2011). This ecosystem is very sensitive to changes in its environment, which is why it has been classified as an indicator of environmental disturbances (Zea, 1991; Bernhard, 2007). For this reason, the temporal variation of the coral cover is considered a good indicator of the health status of coral reefs, since corals are the fundamental constructors of the ecosystem (Birkeland, 1997; Hughes et al., 2010).
Despite their value, coral reefs have suffered extensive degradation in recent decades and about 75 % of them are under threat as a result of anthropogenic and natural disturbances (Burke et al., 2011). Factors such as overfishing, destructive fishing, pollution, high rates of sedimentation, disease, bleaching, and climate change, are the most important causes of deterioration identified globally and nationally, including the San Bernardo’s islands area, in the Colombian Caribbean (Birkeland, 1997; Díaz et al., 2000; Wilkinson and Souter, 2008; Burke et al., 2011).
Although some studies have been carried out in the coral reefs of the San Bernardo archipelago since 1975 (Erhardt and Meinel, 1975; Duque and Gómez, 1983; Prahl and Erhardt, 1985; Díaz et al., 2000; López-Victoria and Díaz, 2000; Garzón-Ferreira and Díaz, 2003), there are few investigations that have evaluated the dynamics of the reefs in the area on a wide spatial and temporal scale. It is highlight the review by Alvarado et al (2011), as well as, the information provided by the National Coral Reef Monitoring System (SIMAC), which in San Bernardo began activities since 2002 (Rodríguez-Ramírez et al., 2010; Navas-Camacho et al., 2011; Bastidas et al., 2014).
From the perspective of management and faced with the problem of climate change, it is important to understand the dynamics of ecosystems over time and detect their changes (Vega-Sequeda et al., 2017). In the coral formations of the San Bernardo archipelago, monitoring of five samplings was carried out between 1989 and 2010, within the framework of the Monitoring and Monitoring Program for Marine Natural Ecosystems of the Environmental Management Plan of the Coveñas Terminal of Ecopetrol S.A. (the oil company Colombian state), in which the substrate coverage and the composition, abundance and coral diversity were evaluated. Additionally, sampling campaigns were carried out in 2013 and 2015. This research analyzed the temporal changes in the coverage and composition of the coral formations, as well as the assembly of scleractinian corals in the San Bernardo archipelago between 1989 and 2015.
STUDY AREA
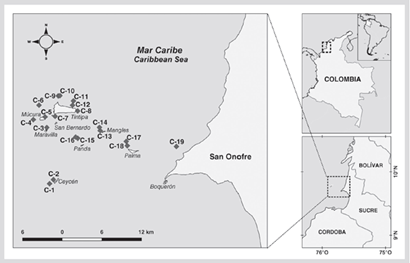
The San Bernardo archipelago is located off the coast of the department of Sucre in the Colombian Caribbean (Figure 1). This archipelago is made up of 12 islands and 4 islets, highlighting the artificial islet of Santa Cruz del Islote as the one with the largest native human population, which derives its livelihood mainly from artisanal fishing and to a lesser extent from tourism (Duque-Rico and Torres-Gómez, 2011). The submerged area is part of the Corales del Rosario and San Bernardo National Natural Park (PNNCRSB) which covers a protected marine area of 120 000 ha (Zarza-González, 2011).
The climate of the region is tropical, with a dry season (December-April) with greater influence from the NE trade winds, a slight decrease in water temperature (27 °C) and an increase in the action of the waves; as well as a rainy season (May-November), when the trade winds weaken, the water is warmer (29 °C), the rains are frequent, and the discharges from the Sinú River that increase turbidity (Pujos et al., 1986; Patiño and Flores, 1993; Andrade-Amaya, 2001; Gutiérrez-Moreno et al., 2011).
The San Bernardo archipelago has a partially emerged reef complex with patches, fringing reefs, and coral banks that occupy an area of approximately 213.3 km², of which 134.5 km2 corresponds to living cover, presenting greater development on the northern flanks and the west. The most prominent formations in this sector are found to the north and northwest of Mangle Island, to the north of Tintipán Island, and on the north and west sides of the Las Nubes, Minalta, and Julián lowlands (Díaz et al., 2000).
The coral reefs of the San Bernardo Islands have suffered strong anthropogenic impacts since the 1980s. These have been attributed to epizootic coral diseases, sedimentation and turbidity from the Sinú River (López-Victoria and Díaz, 2000; Alvarado et al., 2011), dynamite fishing and overfishing, coastal development, uncontrolled tourism, construction on islands, and extraction of corals for handicrafts (Ramírez, 1990; Díaz et al., 2000; Garzón-Ferreira and Díaz, 2003; Alvarado et al., 2011).
MATERIALS AND METHODS
Field phase
The data from the monitoring carried out in 1989, 1991, 1999, 2005, and 2010, documented by Ramírez (1990, 1992, 2000, 2010) and Invemar (2005) were used. Initial monitoring evaluated 11 stations, in 1999 they were expanded to 18, and in 2005 one more was added to complete 19 stations (Figure 1) that are between 4 and 12 m deep. For each monitoring, the historical geographic coordinates of each point were followed. The stations are arranged around the islands Ceycen (C-1 and C-2), Maravilla (C-3), Múcura (C-4 to C-6), Tintipán (C-7 to C-12), Mangle (C-13 and C-14), Panda (C-15 and C-16), Palma (C-17 and C-18), and Cabruna (C-19). At each station, the substrate coverage by algae, corals (by species), other invertebrates (sessile polychaetes, sponges, and anemones), and abiotic substrate were estimated by the intercept point method in the transect. At each site, two non-fixed transects of 75 m were extended, scoring every 10 cm the component of the substrate that was underneath until completing 1500 points.
In 2013 and 2015, the substrate categories described by Garzón-Ferreira et al. (2002) with some modifications: algae, hard corals (by species), sponges, soft corals (gorgonians and zoanthids), other invertebrates, and abiotic substrate. Additionally, in 2015 the presence of coral diseases and bleaching was recorded based on Sutherland et al. (2004) and Raymundo et al. (2008).
Data for the sea surface temperature and the index “degrees of warming weeks” (Degree Heating Weeks - DHW) for 2015 were obtained from the NOAA Coral Reef Watch (2020) satellite databases. The DHW index series shows the amount of heat stress accumulated in corals during the last 12 weeks. This index considers the “tolerance threshold” for coral bleaching, which for the Colombia Caribbean is 29.4 °C. A DHW greater than 4 °C-weeks has shown significant whitening and values above 8 °C-weeks have caused massive bleaching and associated mortality (Liu et al., 2018).
Analysis of the information
To describe the coral assemblage, the measures of specific richness (S), Shannon-Wienner diversity (H’), and Pielou uniformity (J’) were calculated, calculating the average, standard error, and coefficient of variation per station. It is necessary to take into account that in the results of the monitoring before 2013 Agaricia sp. grouped A. agaricites and A. tenuifolia, Orbicella annularis integrated O. faveolata and O. Franksi, and Siderastrea sp. included S. siderea and S. radians.
The temporal variation patterns (between years) of the coral assemblage were represented by Bootstrap averages in Multidimensional Scaling graphs or multivariate MDS and their respective Boostrap intervals of 95 %, with 100 repetitions per group and a coefficient of Minimum correlation of 0.99 (Clarke and Gorley, 2015). To determine possible significant differences in the structure between years, a PERMANOVA with 999 permutations was applied (Anderson, 2001). The corals that made the greatest contribution to the dissimilarity between groups were defined by SIMPER analysis (Clarke, 1993). When making comparisons at the local and national level, differences derived from field evaluation methods were obviated. Thus, the comparison and interpretation are limited to the coverage values of the main categories of the substrate and the abundance of coral species.
RESULTS
Historical characterization
Between 1989 and 2010, in the coral formations of the San Bernardo archipelago, the abiotic substrate was the most conspicuous component. However, between 1989 and 2015 there was a decrease close to 45 %. During the 1990s, the algal component presented a reduction in its coverage, being 1999 the only year in which it was below the corals. However, as of 2010, there was an increase, reaching a maximum in 2013, when it became the predominant component.
Regarding the coral component, between 1989 and 1991 it presented low coverage (less than 20 %). Starting in 2005, there was a gradual increase in coverage, which in 2015 was close to 30 %. However, in 2005 there was a 6 % reduction in coverage compared to 1999 (Figure 2).
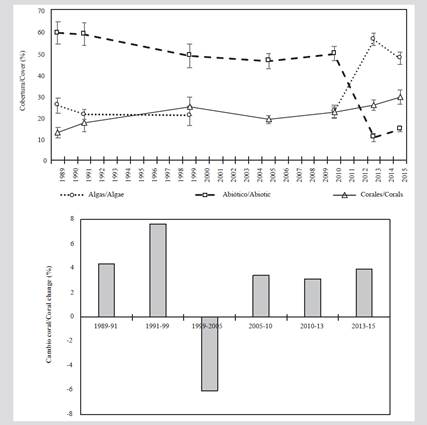
Figure 2 A) Average cover (± standard error) of coral, algae, and abiotic substrate. B) The rate of change in coral cover between 1989 and 2015 in the San Bernardo archipelago.
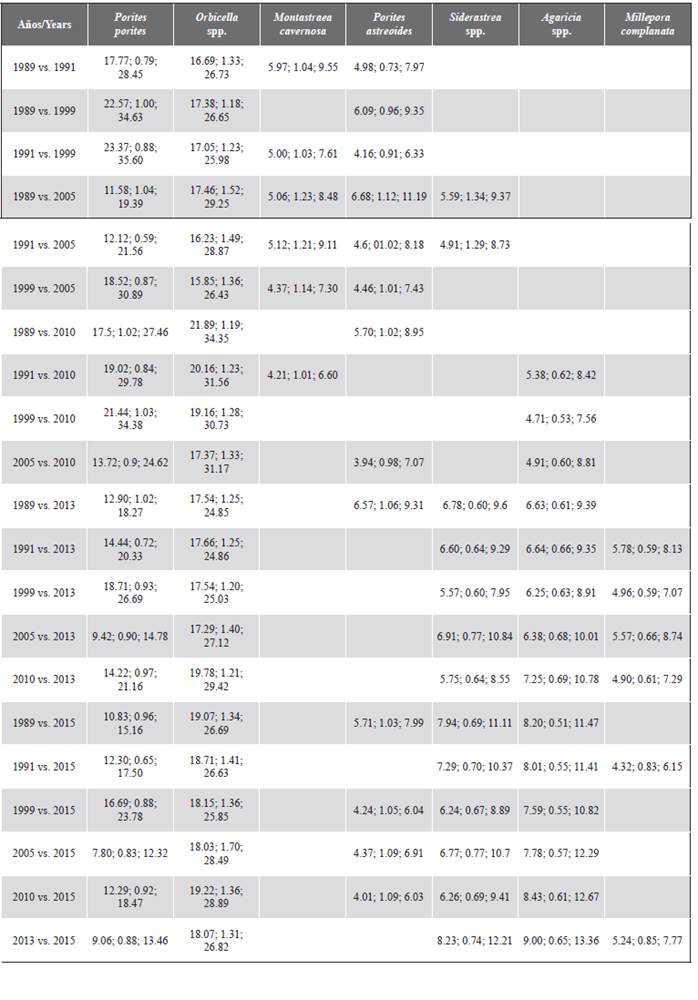
Table 1 shows the coverage of each coral taxon recorded in each of the years. Since 1989, the corals that generally registered the highest relative abundance in the study area were Orbicella spp. and Porites porites, followed by Agaricia spp., P. astreoides and Siderastrea spp. The historical contribution of the Orbicella genus stands out, which was greater than 35 %, as well as its coverage, which increased over time. Additionally, in the decrease in coverage in 2005, the most affected genera were Colpophyllia, Porites, and Pseudodiporia.
Table 1 Percentage of mean coverage
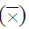 and standard error (SE) of corals in the San Bernardo archipelago between 1989 and 2015.
and standard error (SE) of corals in the San Bernardo archipelago between 1989 and 2015.
At a spatial level, a pattern of greater algae cover, and generally less coral cover, as evidenced in sectors that are closer to the continent such as Palma (C-18) and Cabruna (C-19), especially in 2015 (Figure 3). Corals of the genera Agaricia and Porites were abundant at these stations. On the contrary, stations C-6 (Múcura), C-11 (Tintipán) and C-14 (Mangle), were among the sites that historically registered the highest values of coral cover, in which the genus Orbicella was the most abundant (Tables 1 and 2).
Table 2 Relative coral cover (%) and depth (m) in each of the stations evaluated in the San Bernardo archipelago between 1989 and 2015.
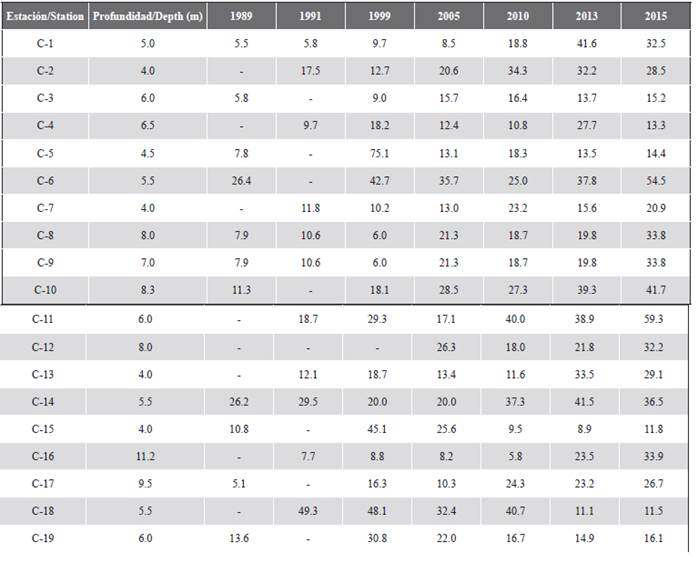
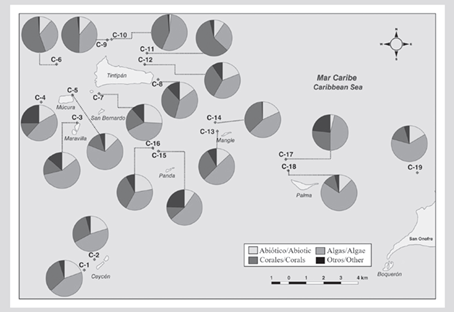
Figure 3 Coverage of the substrate components (%) in each of the stations in the San Bernardo archipelago area in 2015. C-1 and C-2 correspond to the Ceycen sector; C-3 to Maravilla; C-4, C-5, and C-6 to Múcura; C-7, C-8, C-9, C-10, C-11, and C-12 to Tintipán; C-13 and C-14 to Mangle; C-15 and C-16 to Panda; C-17 and C-18 to Palma, and C-19 to Cabruna.
According to the Shannon-Wiener (Ln) index, the average diversity historically has been greater than 1.6 nits, except for the years 1999 and 2010. The low diversity in these two years is related to less uniformity since they were the only periods in which the latter index was less than 0.6. The greater diversity in 2015 and 2005 was more due to greater uniformity (J’ׯ > 0,69), while in 1989 and 1991 it was more the result of greater wealth (Sׯ > 13; Figure 4).
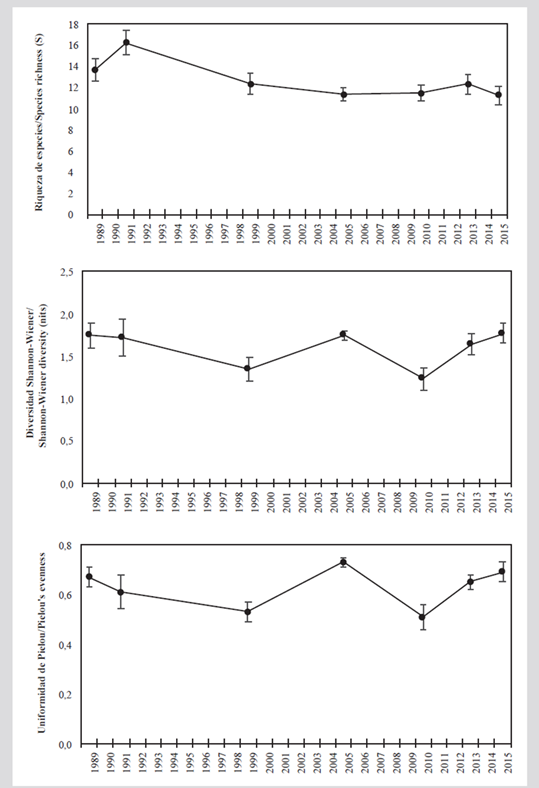
Figure 4 Average diversity measurements (± standard error) of the coral assemblage in the San Bernardo archipelago between 1989 and 2015. A) Species richness. B) Shannon-Wiener diversity (nits). C) Pielou uniformity.
The structure (composition and abundance) of the coral assemblage showed significant temporal differences (PERMANOVA, F(6,116) = 2.19; P = 0.001). Pairwise t-tests indicated that particularly 2015 and 2013 were different from the other years (P < 0.05), but not between them, and that 1989 also presented significant differences with 2005 (Table 3), as evidenced by the graph of averages Bootstrap (Figure 5).
Table 3 Comparison between years of the statistical results of the t-test (probability), using 999 permutations. The asterisk indicates where significant differences were recorded.
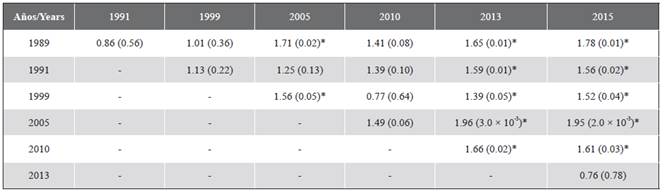
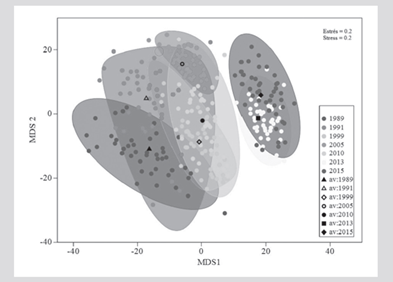
Figure 5 Bivariate graph of Bootstrap averages of the coral assemblage between years. The circles indicate the average of each repetition (transects in a year), the black markers represent the general average of each year and the limits of the geometric. Figures correspond to the Bootstrap region of 95 % confidence.
The SIMPER test revealed that the corals Orbicella spp., P. porites, Agaricia spp., Siderastrea spp. and Millepora complanata contributed at least 70 % of the temporal dissimilarity (Table 4). These species presented greater coverage in 2013 and 2015. In the case of P. porites, although its relative contribution to the temporal dissimilarity of the coral assembly varied between 21.2 % (1999) and 12.8 % (2013), its coverage tends to be cyclical after 2005, when it registered a reduction close to 9 % (Table 1).
Table 4 Taxa that most contribute to the dissimilarity of the coral assemblage between years based on the SIMPER analysis at a cumulative contribution cutoff of at least 70 %. The first value corresponds to the average dissimilarity (%), the second to the consistency (dissimilarity/standard deviation) and the third to the relative contribution (%) of each taxon.
Regarding the presence of signs of coral deterioration, in 2015 bleaching was observed in all stations, except in C-7. The most affected species were Orbicella annularis, O. faveolata, Agaricia tenuifolia, and Siderastrea siderea (Table 5), and in all cases, the bleaching partially affected the colonies. White plague, black band, and dark mole diseases were recorded; this last disease was the most frequent (present in 13 stations; Figure 6) and mainly affected S. siderea coral.
Table 5 Coral species observed with bleaching in the stations evaluated in the San Bernardo archipelago in 2015.
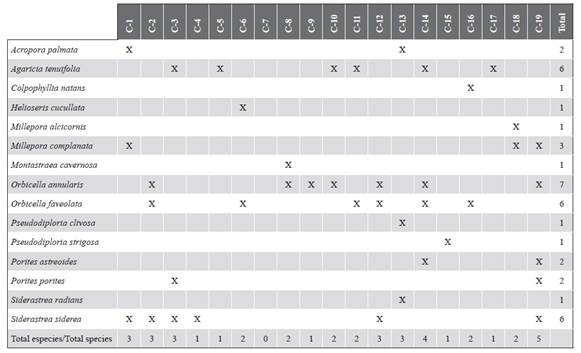
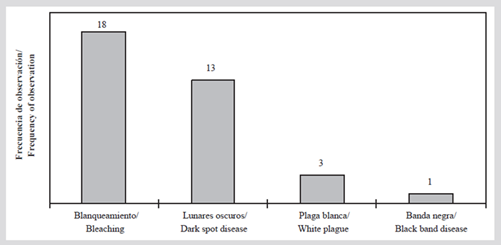
Figure 6 Number of stations with the presence of coral diseases and bleaching in the San Bernardo archipelago in 2015.
Figure 7 shows the daily sea surface temperature (SST) during 2015 in relation to the whitening tolerance threshold of 29.4 °C. Starting in June, temperatures that exceeded the threshold were recorded and the highest values (30.6 °C) were recorded in July and September. During monitoring in August 2015, a DHW of 1.5 °C-weeks was recorded, indicating that the corals were experiencing low heat stress. Subsequently, between October and December, DWH values were recorded between 4 and 8 °C-weeks, indicating high thermal stress that can cause significant bleaching.
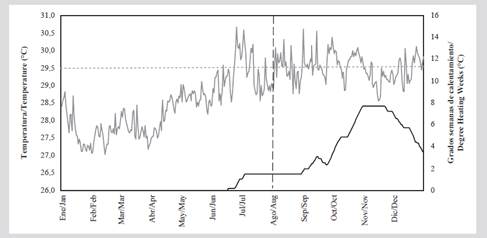
Figure 7 Sea surface temperature (°C, gray line) and DHW thermal stress (°C-weeks, solid black line) between January and December 2015. NOAA satellite data for Colombia, resolution 5 km (NOAA Coral Reef Watch, 2020). The red dotted line indicates the NOAA whitening tolerance threshold (29.4 °C). The vertical dotted line indicates the time when the follow-up was performed.
DISCUSSION
Since the 1980s, the coral formations of the Colombian Caribbean, including those of the Corales del Rosario and San Bernardo National Natural Park (PNNCRSB), have presented changes in the dominance of benthic components, coral composition, mass mortality of organisms, decrease of coral cover, bleaching events and increased disease prevalence (Werding and Sánchez, 1979; Duque and Gómez, 1983; Alvarado et al., 1986, 2011; Cendales et al., 2002; Garzón-Ferreira and Díaz, 2003; Rodríguez-Ramírez et al., 2010; Jackson et al., 2014).
In the PNNCRSB and specifically in the islands of San Bernardo, fishing on hydrobiological resources (Martínez-Viloria et al., 2011), tourist activity (Mendoza et al., 2011), use of biological material for the elaboration of crafts and souvenirs (Ordosgoitia and Zarza-González; 2011), and coral bleaching (Gómez-Campo et al., 2011) have been identified as the main threats.
Other local threats that can be added are the possible influence of sediments and freshwater from the Sinú River and hydrocarbons from the transit of tourist boats, and accidental spills due to activities associated with the Coveñas maritime terminal. The influence of freshwater is evidenced in the alkalinity concentrations both in the rainy season (107.5 to 116.6 mg/L) and in the dry season (119.2 to 130.8 mg/L) of 2013 in San Bernardo (Hyser, 2014). These values are below the ideal limits (175-200 mg/L) and even acceptable (124-225 mg/L) established for the culture of corals (Borneman, 2008), which limits calcification and reduces the rate of growth and skeletal density (Borneman, 2008; Hurtado, 2015).
In the case of hydrocarbons, the results of Betancourt-Portela et al. (2011), who are part of the monitoring carried out by Invemar’s REDCAM, found concentrations below the international reference level of 10 µg/L in the PNNCRSB. Likewise, this document showed that since 2003 all samplings have registered a low level of contamination that does not represent a risk to aquatic organisms. There is also no evidence that the spills that have occurred in the Gulf of Morrosquillo have come to affect the coral formations of the San Bernardo archipelago (Carsucre, 2014).
In the San Bernardo archipelago, between 1989 and 1991 there was a low coral cover (< 20 %) and high values of the abiotic substrate (> 50 %). which were possibly associated with several of the events indicated. One of the events with the greatest impact was the mass death of the dominant coral species at that time, A. palmata and A. cervicornis, due to the white band disease, which decimated their populations in Colombia and other regions of the Caribbean (Díaz et al., 2000; Porter et al., 2001; Garzón-Ferreira and Díaz, 2003; Sutherland et al., 2004; Green et al., 2008).
Before this event, Erhardt and Meinel (1975) and Erhardt and Prahl (1985) mention the presence of large coral patches, as well as extensive hedges of the genus Acropora mainly on the reef crest and numerous colonies of mixed corals in the leeward. Likewise, in Ceycen, they observed the dominance of foliaceous corals at the edge of the slope, which was replaced by massive corals from 12 m depth. Later, in the monitoring results of 1989, Ramírez (1990) highlights the coral deterioration, since the dead coral (16.4 %), which became part of the abiotic substrate, was higher than the coral cover (12.7 %) and was the dominant component of the substrate. The later changes between this component and the algae were due in part to the change of researchers in the 2013 campaign. During the follow-ups between 1991 and 2010, the numerous coral skeletons, despite being colonized by algae, were recorded as an abiotic substrate and in 2013 as algae. Thus, the dominance of this last component may have been present since the 1990s.
The increase in the coverage and dominance of the algae, which persists today, has been documented in other reefs in Colombia and the Caribbean since 1990, evidencing a change of phase, where the reefs are dominated by algae and not by corals (Done, 1992; Hughes, 1994; Díaz et al., 2000; Garzón-Ferreira and Díaz, 2003; Díaz-Pulido et al., 2004; Rodríguez-Ramírez et al., 2010; Bastidas et al., 2014; Jackson et al., 2014). The results of this monitoring coincide with this condition, since historically, except for 1999, the cover of algae was higher than that of coralline. This scenario shows the deterioration process that the reefs of the San Bernardo archipelago have faced in recent decades as a consequence of disturbance factors such as coral diseases, bleaching, climate change, mass tourism, overfishing, pollution, and coastal development, among others occurring in other reefs around the world (Hughes, 1994; McClanahan and Mutinga, 1998; Buddemeier et al., 2004; Díaz-Pulido et al. 2004; Rodríguez-Ramírez et al., 2010; Alvarado et al., 2011).
Additionally, the reduction in coral cover recorded in 2005 was possibly associated with the massive bleaching that occurred that same year, which was one of the most severe events and produced coral mortalities in the Greater Caribbean (Wilkinson and Souter, 2008; Eakin et al., 2010). In the reef formations of the archipelago and surrounding areas, this event severely affected between 1 and 70 % of the corals in the first 12 m of depth, with an associated mortality of 5 % (Rodríguez-Ramírez et al., 2008; Gómez-Campo et al., 2011).
However, bleaching could be a determining factor in coral mortality, threatening the future of reefs, considering that these events are increasingly frequent (Hughes et al., 2017, 2018; Oliver et al., 2018). Additionally, the adaptive response that corals can develop after bleaching is too slow to keep up with the current speed of climate change (Fabricius et al., 2007; Ainsworth et al., 2016).
For the Greater Caribbean, Jackson et al. (2014) documented a general trend towards a reduction in coral cover, on average close to 20 %, between the 1980s and 2011. This same pattern was observed in the reefs of the Colombian Caribbean (Díaz et al., 2000; Alvarado et al., 2011). According to information from the SIMAC monitoring program, coral cover in five reef areas was relatively stable from 1998 to 2004 (Rodríguez-Ramírez et al., 2010). Subsequent results, until 2013, suggest that the areas of Santa Marta and the islands of San Bernardo remain stable, while in the Rosario Islands, San Andrés, and Urabá Chocoano there is a downward trend (Bastidas et al., 2014). Similar to what was reported by SIMAC, in the present monitoring in the San Bernardo archipelago, the corals showed a general trend towards stability between 1989 and 2010.
The richness of coral species observed in this monitoring (40 species) is close to that documented in the study area of 49 and that of other regions such as San Andrés and Urabá Chocoano, both with 41 species, Providencia with 49 species, the Rosario Islands and Santa Marta, both with 53 species (Prahl and Erhardt, 1985; Díaz et al., 2000). However, this information should be taken with caution, since the evaluation was restricted to the first 12 m, which could exclude other habitats and coral formations.
Historically, the corals with the greatest coverage and that contributed the most to the coral assembly were Orbicella spp., Porites porites, Agaricia spp., P. astreoides and Siderastrea spp., which is why they can be considered today as the main constructors of the coral formations studied. This scheme is somewhat consistent with what has been documented by other studies (Alvarado et al., 1986; Cendales et al., 2002; Rodríguez-Ramírez et al., 2010; Alvarado et al., 2011) in the coral formations surrounding Barú and the Rosario Islands.
According to the records of Erhardt and Meinel (1975) and Erhardt and Prahl (1985), before the 1980s, although the Acropora genus was abundant, they also described the presence of numerous coralline species homogeneously distributed in the archipelago. However, P. porites were more commonly observed in the reef lagoon intermixed with Millepora spp. The colonies of S. siderea and P. astreoides, whose diameter was less than 30 cm in diameter, were found mainly as rhodoliths in an unstable substrate. Additionally, in some areas protected from waves, A. tenuifolia was dominant in patches at 6 m depth. On the other hand, massive colonies of Orbicella and Colpophyllia were abundant at depths greater than 12 m.
As a consequence of the mortality of acroporids on Caribbean reefs, including Colombia, Bruckner (2002), Cendales et al. (2002), and Green et al. (2008) state that there was a change in the composition of the coral community, in which pioneer species with opportunistic strategies such as A. tenuifolia, P. astroides, and slow-growing species with a high survival rate such as those of the genus Orbicella and Siderastrea are taking the place of the acropóridos. These changes have resulted in the presence of coral species capable of living under stressful conditions. The increase in coral cover in 2013 may be associated with the change in researchers in this follow-up. However, there could be a contribution by the growth of these species with effective life strategies, although there are no measurements that demonstrate the growth of these colonies during this period.
Throughout the monitoring, the coverage of acroporids was very low, less than 1 %. This suggests that in general, in the evaluated area, the populations of this group have had a low recovery. Studies carried out in different years focused on this coral genus in the Colombian Caribbean, concluded that, despite having a high growth rate, there is no evidence of its real recovery (Díaz et al., 2000; Garzón-Ferreira et al., 2004). Additionally, although García-Urueña and Garzón-Machado (2020) documented different responses in their condition according to the species and its location, the genus Acropora in the San Bernardo archipelago did not show signs of improvement, especially A. cervicornis.
Considering the location of the stations within the archipelago, greater coverage of algae was evidenced, and generally less coral coverage, in the sectors that are closer to the continent such as Cabruna and Palma. In these sectors, there is a greater influence of disturbance factors of human origin, so that these coral formations probably face a continuous process of threats due to the activities that take place in the coastal zone and that lead to deterioration. Indicators such as a decrease in coral cover throughout the monitoring were recorded in several of the stations in these sectors (C-15, C-18, and C-19). The continuous exposure of these coral formations to tensors that cause coral deterioration could limit their capacity for recovery, threatening their persistence (Hughes, 1994; Buddemeier et al. 2004; Hughes et al., 2010; Burke et al., 2011). This deterioration affects the structure and dynamics of the ecosystem and represents a permanent threat to the maintenance of the goods and services derived from it.
On the contrary, there are coral formations that showed better state conditions such as C-6, C-11, and C-14, located to the N and NW of the Múcura, Tintipán, and Mangle islands, respectively. In several of these stations, a greater presence of juvenile corals and an increase in coral cover were observed over time. These stations could be among the best-conserved places in the archipelago and where there is a good probability of increasing the presence of juvenile corals. Therefore, special attention should be paid to their management and protection, since they could be key in the maintenance of the coral communities in the area.
Coral reefs have a narrow range of thermal tolerance, between 18 and 36 °C with an optimal range between 25 and 29 °C (Stoddar, 1969; Hubbard, 1997; Raymundo et al., 2008), so this ecosystem is extremely susceptible to heat stress (Harvell et al., 2007). In 2015, the coral formations faced stress caused by the high temperatures accumulated months ago, which was reflected, in part, in the presence of bleaching in 18 of the 19 stations evaluated and the registration of some diseases. However, its impact was low, as the colonies were only partially affected. However, at the end of 2015 and in 2016 a massive global bleaching event was registered, associated with high temperatures, which severely affected the reefs of the Western Pacific and in Colombia, it was considered a low-level event (Hughes et al., 2017, 2018; Oliver et al., 2018).
From the perspective of resilience, the protection that national parks can provide is only one part of the solution for the conservation of ecosystems, since this must be accompanied by other mechanisms such as strong management measures (Hughes et al., 2010) and ecological restoration (Pizarro et al., 2014). Likewise, management actions on local impacts such as overfishing, tourist activity, degradation of water quality, pollution, deforestation, among others, are essential to stop the deterioration and begin to protect coral reefs to adapt to environmental changes and thus increase their recovery from climate change (Wooldridge et al., 2005; Hughes et al., 2007, 2010; Knowlton and Jackson, 2008). This goes hand in hand with the continuity of monitoring programs to generate information on the state, trends, and changes of marine ecosystems.
CONCLUSIONS
This long-term evaluation showed an opposite dynamic and a great variation in the cover of the abiotic substrate and algae, the latter group being the most abundant component of the seabed since 2013 and corresponding to the general panorama observed in the coral reefs at the world level. The coral assemblage presented a stable trend between 1989 and 2010. However, the temporal dissimilarity registered in 2013 and 2015 with the other years was due to the greater coverage of the corals Orbicella spp., P. porites, Agaricia spp., Siderastrea spp., and M. complanata, whose life strategies, except for M. complanata, have allowed them to become the main builders of the coral formations of the San Bernardo archipelago. The temporal dynamics of the main components of the substrate are a consequence of the effect of natural and anthropogenic disturbances to which these reefs have been subjected in recent decades. However, at a spatial level, great variability of coralline covers can be appreciated, where reefs located on the Múcura, Tintipán, and Mangle islands stand out, presenting better state conditions. Additionally, the higher frequency of observation of bleaching in 2015, which coincided with the record of high temperatures, shows that SST is an important disturbing factor that is currently affecting coral formations.





![Diet and trophic niche breadth of the neotropical cormorant [Nannopterum brasilianus (Gmelin, 1789)] in the Flora and Fauna Sanctuary Ciénaga Grande de Santa Marta, Caribbean Colombia](/img/en/next.gif)





 text in
text in