Short communication
Asynchrony between in vivo and in vitro rabbit embryos*
Asincronía entre embriones in vivo e in vitro en conejo
Assincronia entre embriões in vivo e in vitro em coelho
María-Luz García1
*
Rafik Belabbas2
Raquel Muelas1
Ivan Agea1
María-José Argente1
1Centro de Investigación e Innovación Agroalimentaria y Agroambiental (CIAGRO-UMH), Miguel Hernández University, Ctra de Beniel Km 3.2, 03312 Orihuela, España.
2Laboratory of Biotechnologies related to Animal Reproduction, Institute of Veterinary Sciences, University Blida, B.P 270, road of Soumaa, Blida, 09000, Algeria.
Abstract
Background:
Comparative features of embryos developed under in vitro and in vivo conditions are particularly important in designing embryo transfer procedures that fulfil embryo-recipient synchronization requirements.
Objective:
To determine the degree of asynchrony in rabbit embryo development between cultured and in vivo embryos.
Methods:
A total of 55 non- lactating multiparous female rabbits were used. Embryos were classified as 16-cells or early morulae at 48 hours post-coitum (hpc). Embryos were cultured during 30 or 32 h and embryo development was compared with in vivo embryos of 72 hpc. In vitro and in vivo embryos at 72 hpc were classified as early or compacted morulae. Bayesian statistics was used. Difference between in vivo and in vitro embryos and the actual probability of the difference between the in vivo and in vitro embryo higher than zero (P) was estimated.
Results:
The percentage of compacted morulae was higher in in vivo embryos than in in vitro embryos with +6 h of asynchrony (73.5 and 32.8%, P=1.00). But the percentage of compacted morulae was similar with +8 h asynchrony.
Conclusions:
In vitro embryos delay their development by + 8 hours compared to in vivo embryos.
Keywords: assisted reproduction; embryo culture; embryonic asynchrony; embryonic development; embryo synchronization; embryo transfer; in vitro embryo production; in vivo embryo production; morulae; rabbits
Resumen
Antecedentes:
El desarrollo comparativo de embriones producidos in vitro e in vivo es particularmente importante para el diseño de procedimientos de transferencia de embriones cuando se requiere sincronización entre el embrión y la hembra receptora.
Objetivo:
Determinar el grado de asincronía en el desarrollo embrionario entre embriones in vivo y cultivados.
Métodos:
Un total de 55 conejas multiparas no lactantes fueron utilizadas. Los embriones se clasificaron en 16 células o mórulas tempranas a las 48 horas después del coito (hpc). Los embriones se cultivaron durante 30 ó 32 horas y el desarrollo embrionario se comparó con embriones de 72 hpc obtenidos in vivo. Los embriones in vitro e in vivo a 72 hpc se clasificaron como mórulas tempranas o compactas. Se utilizó estadística bayesiana. Se estimó la diferencia entre embriones in vivo e in vitro y la probabilidad de que la diferencia sea superior a cero (P).
Resultados:
El porcentaje de mórulas compactas fue mayor en embriones in vivo que en embriones in vitro con +6 horas de asincronía (73,5 y 32,8%, P=1,00), pero el porcentaje de mórulas compactas fue similar con asincronía de +8 horas.
Conclusión:
Los embriones cultivados retrasan +8 horas su desarrollo en comparación con los embriones in vivo.
Palabras clave: asincronía embrionaria; conejo; cultivo de embriones; desarrollo embrionario; mórula; producción de embriones in vitro; producción de embriones in vivo; reproducción asistida; sincronización de embriones; transferencia de embriones
Resumo
Antecedentes:
A aquisição do desenvolvimento de embriões produzidos in vitro e in vivo é particularmente importante na concepção de procedimentos de transferência de embriões em que a sincronização entre o embrião e a fêmea receptora é necessária.
Objetivo:
Determinar o grau de assincronia no desenvolvimento embrionário entre embriões cultivados e in vivo.
Métodos:
Um total de 55 coelhos multíparos não lactantes foram usados. Os embriões foram classificados em 16 células ou mórulas iniciais 48 horas de gestação (hpc). Os embriões foram cultivados por 30 ou 32 horas e o desenvolvimento embrionário foi comparado com embriões de 72 hpc obtidos in vivo. Embriões in vitro e in vivo a 72 hpc foram classificados como mórulas precoces ou compactadas. Estatísticas bayesianas foram usadas. A diferença entre embriões in vivo e in vitro e a probabilidade de que a diferença seja maior que zero (P) foi estimada.
Resultados:
A porcentagem de mórulas compactadas foi maior em embriões in vivo do que em embriões in vitro com +6 horas de assincronia (73,5 e 32,8%, P=1,00). Mas a porcentagem de mórulas compactadas foi semelhante com assincronia de +8 horas.
Conclusão:
Embriões cultivados atrasam seu desenvolvimento em +8 horas em comparação com embriões in vivo.
Palavras-chave: assincronia embrionária; coelho; cultura de embriões; desenvolvimento embrionário; mórula; produção in vitro de embriões; produção de embriões in vivo; reprodução assistida; sincronização de embriões; transferência de embrião
Introduction
Rabbit zygotes and early embryos have been successfully developed to blastocysts using different culture media (Saenz-de-Juano et al., 2011). However, these media do not mimic the oviductal environment and in vitro developed embryos differ from their in vivo analogues. Specifically, in vitro cultured rabbit embryos show fewer cells, smaller diameters (Adams, 1970) and morphological signs of degeneration after one day in culture from morulae to early blastocysts (Hegele-Hartung et al., 1988). Thus, embryos undergo a retarded development under in vitro conditions (Carney and Foote, 1990).
The female rabbit has certain physiological and anatomical characteristics that make it especially suitable for the application of embryo reproductive techniques (García, 2018). In vitro embryo culture is an assisted reproductive technique used frequently in embryo biology. The comparative features of embryos that develop under in vitro and in vivo conditions are particularly important for designing embryo transfer procedures that fulfil embryo-recipient synchronization requirements. Therefore, in order to maximize the results of the application of reproductive techniques, it is necessary to accurately determine the degree of delay in embryonic development of cultured embryos. Therefore, the aim of this study was to determine the degree of asynchrony in embryo development between in vitro and in vivo embryos at 72 hpc in rabbits.
Materials and Methods
Ethical considerations
All experimental procedures were approved by the Committee of Ethics and Animal Welfare of the Miguel Hernández University, Spain (Ref: 2019/VSC/PEA/0017).
In vivo embryo production and collection
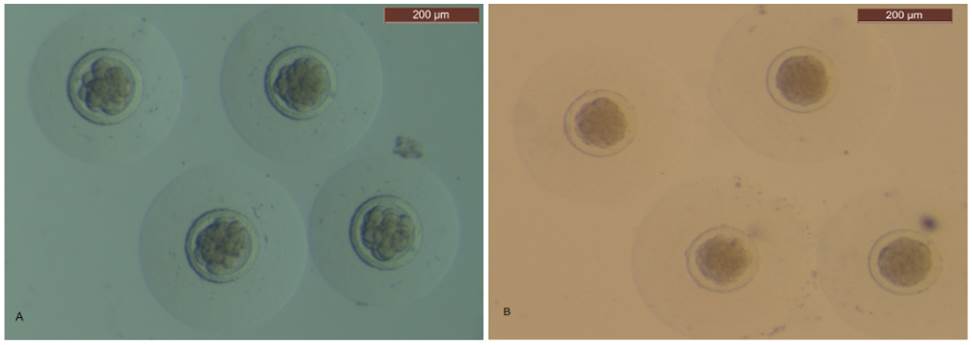
Non-lactating multiparous female rabbits were used (Argente et al., 2019). A total of 41 and 14 does were mated and then slaughtered at 48 and 72 hours post-coitum (hpc) by intravenous administration of 50 mg sodium thiopental/kg body weight (thiobarbital, B. Braun Medical S.A., Barcelona, Spain). The ovaries, oviducts and uterine horns were removed. To recover the embryos, the oviduct and the first third of the uterine horn were flushed with 5 mL Dulbecco phosphate buffered saline (DPBS, Sigma, Alcobendas, Madrid, Spain) supplemented with 0.2% (wt/vol) bovine serum albumin (BSA, Cod. A-3111, Sigma) and 0.2 mL of antibiotic (Penivet 1, Divasa Farmavic, Barcelona, Spain) at room temperature. In vivo embryos were examined. They were considered normal when homogenous cellular mass and spherical zona pellucida and mucin coat was present. A binocular stereoscopy microscope (Leica Mz 9.5-600x, Wetzlar, Germany) was used. At 48 hpc, normal embryos were classified as 16-cells or early morulae (Figure 1A). At 72 hpc, normal embryos were classified as early morulae or compacted morulae (Figure 1B).
Embryo culture
Normal embryos at 48 hpc from each doe were cultured in a one-well 4-well embryo culture dish (NUNC A/S, Thermo Fischer Scientific, Denmark) containing 1 ml culture media (TCM- 199 supplemented with 10% fetal bovine serum). Culture was performed at 38.5 ºC in 5% CO2 in air saturated humidity. In vitro developed embryos were examined after 30 or 32 h of culture. So, the asynchrony between in vivo and in vitro embryos was +6 h or +8 h. Then, embryos were classified as early morulae or compacted morulae. A total of 20 and 21 females were used to culture their embryos with an asynchrony of + 6h and + 8h, respectively.
Statistical analyses
Early morulae and compacted morulae were expressed as a percentage of normal embryos. Differences in asynchrony between in vivo and in vitro embryos were estimated with a model including the effects of asynchrony (in vivo embryos, and in vitro embryos with +6 h and +8 h of asynchrony). Analysis was performed using Bayesian methodology. Bounded uniform priors were used for all effects. Residuals were, a priori, normally distributed with mean 0 and variance Iσ2e. The prior for the variance was also bounded uniform. Features of the marginal posterior distributions for all unknowns were estimated using Gibbs sampling. Inferences were derived from the marginal posterior distributions. Median, difference between in vivo and in vitro embryos (D), and the shortest interval with 95% probability of containing the true value (HPD95%) were provided. The HPD95% showed the precision of the estimation and can be asymmetric around the estimation. The actual probability of D higher than zero (P) was estimated. The Rabbit program developed by the Institute for Animal Science and Technology (Valencia, Spain) was used.
Results
Table 1 shows differences between in vivo and in vitro embryo development. When the asynchrony was +6 h, the percentage of early morulae was lower in in vivo than in in vitro embryos (-40.7%, P = 1). So, the percentage of compacted morulae was higher. An asynchrony of +6 h was not enough for matching in vitro and in vivo embryo development. Nevertheless, the percentage of early morulae (-7.0%, P = 0.68) and compacted morulae were similar between in vivo and in vitro embryos with +8 h of asynchrony.
Table 1 Differences between development between in vivo and in vitro embryos.
| |
In vivo |
In vitro |
D |
HPD95% |
P |
| +6 h asynchrony (N) |
14 |
20 |
|
|
|
| Early morulae |
32.8 |
73.5 |
-40.7 |
-65.0, -16.3 |
1.00 |
| Compacted morulae |
67.2 |
26.5 |
40.7 |
16.1, 66.2 |
1.00 |
| + 8 h asynchrony (N) |
14 |
21 |
|
|
|
| Early morulae |
32.8 |
39.8 |
-7.0 |
-36.0, 18.3 |
0.68 |
| Compacted morulae |
67.2 |
60.2 |
7.0 |
-20.1, 34.1 |
0.68 |
N=number of does; In vivo=median of in vivo embryos at 72 hpc; In vitro=median of in vitro embryos; D=median of the difference between the in vivo and in vitro embryos; HPD95%=highest posterior density region at 95%; P=probability of the difference being ˃0 when D˃0 and probability of the difference being <0 when D<0.
Discussion
The low development of cultured embryos also occurs in cattle (Lonergan et al., 2016) and pigs (Fowler et al., 2018). While embryo culture systems are static, in vivo embryos are exposed to a constantly changing environment as it passes along the oviduct to the uterus. Concomitantly, embryos exhibit changes in physiology and energy metabolism between fertilization and blastocyst (Gardner, 1998). Thus, embryo manipulation and adaptation to in vitro conditions as its requirements change during development lead to a reduction in development (García, 2018). We have determined that asynchrony in rabbits is 8 hours between cultured and in vivo embryos. This result can be used to optimize the design of experiments in which cultured embryos are used.
In conclusion, development was lower in in vitro than in in vivo embryos. Asynchrony between in vivo and in vitro embryonic development was 8 hours.
References
Adams CE. The development of rabbit eggs after culture in vitro for 1-4 days. J Embryol Exp Morphol 1970; 23: 21-34.
[ Links ]
Argente MJ, García ML, Zbyňovská K, Petruška P, Capcarová M, Blasco A. Correlated response to selection for litter size environmental variability in rabbits’ resilience. Animal 2019; 13: 2348-2355. https://doi.org/10.1017/S1751731119000302
[ Links ]
Carney EW, Foote RH. Effects of superovulation, embryo recovery, culture system and embryo transfer on development of rabbit embryos in vivo and in vitro. J Reprod Fertil 1990; 89: 543-551. https://doi.org/10.1530/jrf.0.0890543
[ Links ]
Fowler KE, Mandawala AA, Griffin DK, Walling GA, Harvey SC. The production of pig preimplantation embryos in vitro: Current progress and future prospects. Reprod Biol 2018; 18: 203-211. https://doi.org/10.1016/j.repbio.2018.07.001
[ Links ]
Gardner DK. Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology 1998; 49: 83-102. https://doi.org/10.1016/s0093-691x(97)00404-4
[ Links ]
García ML. Embryo manipulation techniques in the rabbit. New Insights into Theriogenology. Edited by Rita Payan-Carreira; 2018. https://doi.org/10.5772/intechopen.81089
[ Links ]
Hegele-Hartung C, Fischer B, Beier HM. Development of preimplantation rabbit embryos after in vitro culture and embryo transfer: An electron microscopic study. Anat Rec 1988; 220: 31-42. https://doi.org/10.1002/ar.1092200105
[ Links ]
Lonergan P, Fair T, Forde N, Rizos D. Embryo development in dairy cattle. Theriogenology 2016; 86: 270-277. DOI: https://doi.org/10.1016/j.theriogenology.2016.04.040
[ Links ]
Saenz-de-Juano MD, Naturil-Alfonso C, Vicente JS, Marco-Jiménez F. Effect of different culture systems on mRNA expression in developing rabbit embryos. Zygote 2011; 21: 103-109. https://doi.org/10.1017/S0967199411000414
[ Links ]