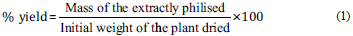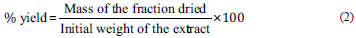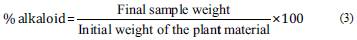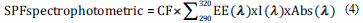Introduction
The use of medicinal plants to treat various diseases is a common practice in Latin American countries. In this sense, a significant increase in Latin American scientific production has been observed in recent years in order to encourage the use of standardized herbal medicines that have proven quality, safety and efficacy [1]. Among the hundreds of families studied, the Cleomaceae family stands out for having numerous biological activities, such as anti-inflammatory, antinociceptive, antibacterial, fever, malaria and diabetes [2].
Tarenaya aculeata is a species belonging to the Cleomaceae family, and there are very few studies in the literature that prove its effectiveness. In the literature, its popular use is mainly reported in the treatment of fever and inflammation [3,4], but there are reports of its use also for the treatment of other disorders, such as diabetes, cholesterol and high blood pressure [5]. Tea from the whole plant is normally administered [3,5], but there are also reports on its use for bathing or with respect to tea from the root or the whole plant [3-5].
Fever is caused by several factors, one of the known causes being the inflammatory process [6]. The inflammatory response consists of an innate system of cellular responses elicited after an injury, which may arise from exposure to heat or cold, schema/reperfusion, blunt trauma, as well as due to the action of infectious organisms [7]. Therefore, evaluating the antibacterial activity of this plant and possible photoprotective effects may be related to the antipyretic and anti-inflammatory capacity of this plant.
Another widely evaluated biological activity in medicinal plants is the antioxidant activity, and as this is the first screening to contemplate the biological potential of T. aculeata, it will be contemplated in our study. The evaluation of antioxidant studies is important because many diseases are caused due to oxidative stress, and medicinal plants are rich in secondary metabolites and can act as free radical scavengers, avoiding numerous diseases, such as cancer, Alzheimer's, heart, kidney and liver diseases, fibrosis, atherosclerosis, arthritis, neurodegenerative disorders and aging [8].
The assessment of photoprotective activity has gained importance among researchers in recent times, mainly due to the number of cases of skin cancer that has been increasing worldwide, caused mainly by ultraviolet radiation [9]. The exposure to this specific wavelength range interacts with unprotected living tissue and causes biochemical and physiological changes, which can result in sunburn, premature aging and increased risk of skin cancer [10]. In this sense, plant extracts have proven to be a great alternative to be incorporated into sunscreens, which become an important strategy in order to prevent the negative damage caused due to overexposure [11].
Since antimicrobial resistance has become one of the main threats to public health in recent years, research directed at discovering new compounds that can act as antimicrobial agents has also been strongly encouraged. Antibiotic resistance compromises the ability of the human immune system to fight infectious diseases and in this sense, plant-based antimicrobial studies have received attention due to their potential for fighting diseases caused by microorganisms (bacteria, fungi, protozoa and viruses), and for their ability to act as anti-infective agents with few side effects [12,13].
However, and due to the various biological activities, that can be found in a plant, a study is also necessary for the evaluation of the possible toxic effects present in the extract. In this sense, the lethality assay involving Artemia salina constitutes a good alternative for preliminary evaluation, since it is a quick, convenient and low-cost test [14]. A. salina is a small halophilic invertebrate belonging to the Crustacean class that has been widely used in applied toxicology research [15].
Chemical characterization studies of plant extracts are essential, as they allow a more rational exploration of Brazilian flora. Secondary metabolites are responsible for numerous biological activities in plants [9,16]. Thus, and due to the possibility of this plant serving as a natural source of effective compounds, the objective of this study is to carry out a phytochemical screening, a study of the content of alkaloids, phenolic compounds and tannins as well as the antioxidant, photoprotective and antibacterial activities of T. aculeata and the toxicity against A. salina.
Materials and methods
Plant material and extraction
Stems and roots of T. aculeata were collected in the municipality of Dourados, MS, Brazil. The plant was identified by a botanist specialising in the Tarenaya genus, Dr. Raimundo Luciano Soares Neto, Federal University of Paraíba. An exsiccate (DDMS 7618) of the plant species was deposited at the DDMS Herbarium, Universidade Federal da Grande Dourados- MS. Registration number of the SISGEN (AD26007).
The plant materials were dried in an oven with air circulation at 50 °C for 72 h and ground using a Wiley mill (Marconi, Brazil). Their respective extracts were prepared by the decoction method. The decoctions were prepared at 2% (w/v) by immersing the plant material in boiling water for 20 min, in an open system, followed by an approximately 10 min wait before performing the filtration. Subsequently, the extract was filtered and a portion was separately frozen and lyophilised (Martin Christ, Alpha 1-2 LD plus, Germany), with a pressure of 0.045 mbar and a temperature of -42 °C, in order to obtain the stem extracts (SE) and root extracts (RE), while another portion of each extract was used to obtain the methanolic stem (SF) and root (RF) fractions. The yields obtained for the stem and root extracts were 15 and 8%, respectively, in relation to the plant dried, and were determined according to eq (1).
In order to eliminate water-soluble compounds and obtain enriched fractions, the remaining aqueous solutions were individually subjected to a solid phase extraction procedure using Amberlite XAD-2 resin, as per the methodology described by Yung An et al. [17] with modifications. In short, a glass column was packed with 100 g of XAD-2 resin (chromatographic bed dimensions: 25 cm h x 3 cm 0), initially conditioned in distilled water. Then, the hydrophobic resin was, successively, loaded with the sample (two L of one of the decoctions), washed with 700 mL of distilled water and the adsorbed compounds eluted with 700 mL of methanol. Finally, the methanolic solutions thereby obtained were concentrated to dryness in a fume hood with exhaustion, and the yield of the methanolic fractions was SF: 6% and RF: 9%, in relation to aqueous extracts, respectively, and were determined according to eq (2):
Chemical composition
Phytochemical screening
All samples were tested for the presence of phytochemicals. The alkaloid screening was performed using the methodology described by Silva et al. [18]. Similarly, the presence of phenolic compounds, flavonoids, glycosides, saponins and tannins in the extracts and fractions was also evidenced by means of qualitative phytochemical tests, which were performed as described by Guterres et al. [19].
Analysis of the alkaloid contents
The analysis of the total alkaloid content was determined according to the methodology described by Oliveira et al. [20]. For this, two g of plant material was reacted with eight mL of 10% acetic acid, prepared in ethanol, for four h, filtered and precipitated with concentrated ammonium hydroxide. The solution was allowed to stand and filtered with dilute ammonium hydroxide. The residue obtained was then dried and its composition was determined according to eq (3):
Determination of phenolic and tannin contents
Stock aqueous solutions at 1.000 μg mL-1 of SE, SF, RE and RF were prepared and used for the quantification of total phenolic compounds and tannins. All analyses were performed in triplicate.
The content of the first group was determined using the Folin-Cionalteau colourimetric method [21]. Briefly, 100 μL of the stock solution was mixed with the Folin-Ciocauteau reagent, distilled water and 20% sodium carbonate and incubated in a dark environment at 23±2 °C for two h. The absorbance was recorded at 760 nm wavelength, using a UV/Vis spectrophotometer (Global Trade Technology, Brazil). This process was carried out for each sample. The analytical curve was prepared with gallic acid at concentrations between 10 and 100 μg mL-1. The results were expressed as mg of gallic acid per g of dried extract or fraction (mg GAE g-1).
The total tannin content was determined using the Folin-Denis spectrophotometric method [22]. The sample (two mL) was mixed with two mL of Folin-Denis reagent and two mL of 8% sodium carbonate. The mixture was left to react for two h and then the absorbance was read at 725 nm. This process was undertaken for each sample. Standard solutions of tannic acid in the concentration ranging from 0.5 to 150 μg mL-1 were used to plot an analytical curve. Results were expressed as mg of tannic acid equivalent per g of dried extract or fraction (mg TAE g-1).
Antioxidant activity against the 2,2-diphenyl-1-picrylhydrazyl radical
The antioxidant activity of the extracts and fractions were evaluated by the DPPH (2,2-diphenyl-1-picrylhydrazryl) radical method [23]. For this, a stock solution of SE, SF, RE and RF was prepared at a concentration of 500 μg mL-1 in a 50% water/methanol solution and, from there, dilutions at concentrations of 240, 120, 60 and 30 μg mL-1 were prepared. To assess the antioxidant potential, two mL of a methanolic DPPH 0.004% solution was added to 100 μL of the stock solution to be tested, and also to each of its dilutions. The samples were incubated in a dark environment and left to react for 30 min at a controlled temperature (25 ± 1 °C). Absorbance was recorded at a wavelength of 517 nm. A 50% water/methanol solution was used as a blank.
The results were expressed in inhibitory concentration (IC50), that is the concentration of the sample required to scavenge 50% of DPPH free radicals [24] and for this, a graph was plotted, considering the Y axis the concentration of the extracts (μg mL-1) and the X axis the percentage of inhibition activity (%). All tests were performed in triplicate.
Photoprotective potential
The analysis of the photoprotective potential was determined by means of a spectral scan of the extracts, the calculation of the critical wavelength and the SPF value. For the analyses of the photoprotective potential, extracts and fractions of stems and roots were used, at a concentration of 200 μg mL-1, obtained from the dilution of stock solutions. The exploratory scans of the solutions were recorded at wavelengths between 200 and 600 nm, with a 1 nm interval, using a UV/Vis spectrophotometer. To calculate the critical wavelength (λc), the intervals from 290 nm to 400 nm were selected and the graph area was calculated and integrated, using the Orgin Pro 2018 software version 9.5. The wavelength was equivalent to 90% of the spectrum area [25,26].
To determine the SPF of the solutions, the samples were read in a UV/Vis spectrophotometer between 290 and 320 nm, with an interval of 5 nm. As a blank of the analysis, distilled water is used for the extracts and fraction samples. The absorbances obtained were substituted in the eq (4), as follows [27]:
Where CF= correction factor of 10; EE= Erythemal effects spectrum; I= Solar intensity spectrum and ABS= absorbance of sunscreen sample. The values of EE x I are constant values that were determined by Sayre et al. [28] and are presented on Table 1:
Table 1 Normalized product function determined by Sayre et al. [28] used to calculate the SPF value.
| Wavelenth (λ nm) | EE x I |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0839 |
| 320 | 0.0180 |
| Total | 1.0000 |
EE: Erythemal effect spectrum; I: Solar intensity spectrum.
Antibacterial activity
The determination of the minimum inhibitory concentrations (MIC) of the SE, SF, RE and RF were ascertained according to the methodology described by Bernardi et al. [29]. For this, seven bacterial strains were tested, four of which were Gram-positive (Enterococcus faecalis ATCC29212; Staphylococcus epidermidis ATCC12228; Staphylococcus aureus ATCC 25232 and Staphylococcus saprophyticus ATCC15305) and three Gram-negative (Burkholderia cepacia NEWP 0059; Escherichia coli ATCC 38731 and Pseudomonas aeruginosa ATCC27853).
Microplates containing 96 wells were used to carry out the assay. They were all filled with 100 μL of Mueller Hinton broth containing different concentrations of the SE, SF, RE and RF (15.62-1000 μgmL-1) and the same volume of bacterial saline solution (0.9%) in Mueller Hinton broth. The microplate was incubated at 36 ± 1 °C for 24 h. Tetracycline (15 μg mL-1) was used as a positive control and distilled water as a negative control. After incubation, the MIC was determined using a spectrophotometer. All analyses were performed in triplicate.
Toxicity test
The toxicity test was performed based on the proposal put forth by Meyer et al. [14], with adaptations. To carry out the toxicity test on A. salina, a stock solution was prepared at a concentration of 1500 μg mL-1, and dilutions at concentrations of 0; 0.5; 1.0; 5.0; 25; 125; 250; 500 and 1000 μg mL-1. 10-mL of each of the solutions was transferred to graduated test tubes and then 10 nauplii of A. salina were added. To perform the toxicity test, the A. salina cysts had been previously incubated for a period of 48 h in a 3% sea salt solution at room temperature and under 100 W light and constant aeration.
After 24 h of exposure, a count of live and dead nauplii was performed, and all those that showed any type of movement when observed near a light source were considered alive. A control was prepared containing only saline water and A. salina. To calculate the mean lethal concentrations (LC50), a graph was plotted considering the percentage of dead nauplii (%) by the extract concentration (μg mL-1), obtaining an equation of the straight line for each of the tested extracts and fractions, and the respective values of a and b. All tests were performed in triplicate.
Statistic analysis
Statistical analyses were carried out only to evaluate the levels of phenolic compounds and tannins present in the extracts and fractions of T. aculeata. For this, these samples were subjected to one-way analysis of variance (ANOVA) and a posteriori Tucker test. The level of significance of the analyses was considered when p < 0.05. These analyses were performed using the free software Bioestat 5.0 [30].
Results
Phytochemical screening
According to Table 2, T. aculeata has a great variety of phytochemicals. Alkaloids, phenolic compounds, flavonoids, glycosides and tannins were found in all extracts and fractions, whereas saponins were detected only in the SE and the RE (Table 2). In general, the classification in relation to the intensity showed that the fractions present the highest levels of secondary metabolites, especially the SF (Table 2).
Table 2 Phytochemical screening of T. aculeata extracts and fractions.
| SE | SF | RE | RF | |
|---|---|---|---|---|
| Alkaloids | + | +++ | + | ++ |
| Phenolic compounds | + | +++ | + | ++ |
| Flavonoids | ++ | +++ | + | + |
| Glycosides | + | +++ | + | ++ |
| Saponins | + | - | + | - |
| Tannins | + | ++ | + | + |
- negative; + positive - low intense; ++ positive medium intense; +++ positive strong intense. SE: Stem extract; SF: Stem fraction; RE: Root extract; RF: Root fraction
Analyses of the alkaloids, tannins and phenolic compounds
T. aculeata stem presented 7.65% of alkaloids, a value higher than the root (1.10%), in the plant material. The content of phenolic compounds and tannins are shown on Table 3. The content of phenolic compounds evidenced a statistical difference between extracts and fractions (p < 0.05), when the same part of the plant was used. Statistical similarity was visualized between the SE and the RE (p > 0.05), showing that these plant parts have similar compound contents when extracted using the same extraction method. The tannin content, however, presented statistical similarity (p > 0.05) among all the analyzed samples, not showing chemical differentiation regarding the tannin analysis, in the extracts and/or fractions.
Table 3 Contents of phenolic compounds and tannins in T. aculeata extracts and fractions.
| Extract | Phenolic compounds (mg GAE g-1) | Tannins (mg TAE g-1) |
|---|---|---|
| SE | 54.13 ± 2.52a | 44.51 ± 0.84a |
| SF | 312.80 ± 2.77b | 44.07 ± 3.84a |
| RE | 41.47 ± 8.57a | 44.55 ± 0.83a |
| RF | 248.80 ± 2.04c | 43.96 ± 3.58a |
Values expressed as mean ± standard deviation. SE: Stem Extract; SF: Stem fraction; RE: Root extract; RF: Root fraction. GAE: Gallic acid equivalent; TAE: Tannic acid equivalent. Averages followed by different letters differ by Tukey test.
Evaluation of the antioxidant, photoprotective and antibacterial activities
Considering the free radical-scavenging capacity using the DPPH reagent, and according to Reynertson et al. [31], the antioxidant activity of a plant can be classified into groups, being considered active (values below 50 μg mL-1), moderately active (values between 50 and 100 μg mL-1), slightly active (values between 100 and 200 μg mL-1) and inactive (values > 200 μg mL-1). The fractionation of the samples affected the results of the antioxidant analysis. Thus, the SF were considered active and the RF moderately active, whereas the SE and the RE were considered inactive (Table 4). The use of the XAD-2 resin caused an increase in the levels of compounds that resulted in a greater antioxidant effect present in the evaluated fractions.
Table 4 Antioxidant and photoprotective potentials, critical wavelength and toxicity assessment of T. aculeata extracts and fractions.
| IC 50 (μg mL-1) | SPF value | Λc (nm) | LC50 in Artemia salina (μg mL-1) | |
| SE | 347.06 ± 0.01 | 2.39 ± 0.20 | 364.67 ± 3.05 | 602.58 ± 3.99 |
| SF | 34.71 ± 0.67 | 16.58 ± 0.06 | 370.67 ± 0.58 | 569.58 ± 11.67 |
| RE | > 500 | 1.49 ± 0.08 | 350.00 ± 1.00 | > 1500 |
| RF | 85.39 ± 3.08 | 17.73 ± 0.03 | 371.67 ± 0.58 | 289.23 ± 8.87 |
Values expressed as ± standard deviation. SE: Stem extract; SF: Stem fraction; RE: Root extract; RF: Root fraction. IC 50 : Inhibitory concentration; SPF: Sun protection factor; Ac: Critical wavelength; LC 50 : Lethal concentration.
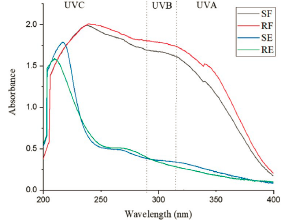
The results of the molecular absorption analysis (Figure 1) showed that the extracts and fractions present UV absorption. The RF showed the highest absorption values for both UVB and UVA regions, followed by the SF. The SPF value and critical wavelength results are contained on Table 4.
According to Machado et al. [32], when plant extracts present MIC below 10 μg mL-1, the antibacterial activity is considered excellent; good when it varies from 10 to 100 μg mL-1, moderate from 100 to 500 μg mL-1, weak when it varies from 500 to 1000 μg mL-1 and inactive for extracts above 1000 μg mL-1. Thus, the tested extracts and fractions showed good to moderate antimicrobial activity against all Gram-positive and Gram-negative bacteria tested (MIC < 1000 μg mL-1), with broad-spectrum action being evidenced for all extracts and fractions (Table 5).
Table 5 Mínimum Inhibitory Concentration (MIC) of T. aculeata extracts and fractions.
| MIC (μg mL-1) | ||||
|---|---|---|---|---|
| Microorganism | SE | SF | RE | RF |
| Burkholderia cepacia - | 250 | 125 | 250 | 125 |
| Enterococcus faecalis + | 250 | 125 | 250 | 125 |
| Escherichia coli - | 125 | 62.5 | 125 | 62,5 |
| Pseudomonas aeruginosa - | 125 | 31.25 | 125 | 31.25 |
| Staphylococcus epidermidis + | 250 | 125 | 250 | 125 |
| Staphylococcus aureus + | 250 | 62.5 | 250 | 62.5 |
| Staphylococcus saprophyticus + | 500 | 250 | 500 | 250 |
+ gram-positive bacteria; - gram-negative bacteria; SE: Stem extract; SF: Stem fraction; RE: Root extract; RF: Root Fraction
Toxicity test
In relation to the toxicity test the classifications were based on those proposed by Sandoval et al. [33] and can be classified as non-toxic (LC50 greater than 1000 ¡g mL-1), low toxic (concentrations between 500 and 1000 μg mL-1), moderate toxicity (LC50 between 100 and 500 μg mL-1) and high toxicity (below than 100 μg mL-1). Thus, the RE can be classified as non-toxic, the SE and the SF are of low toxicity and the RF was the only sample to be considered of medium toxicity (Table 4).
Discussion
Several studies have already reported the presence of alkaloids, phenolic compounds, flavonoids, glycosides, saponins and tannins in Tarenaya species, being common in the Cleomaceae family [2,34]. Phenolic compounds were identified and quantified by high performance liquid chromatography (HPLC) in the ethanolic and aqueous extracts of Tarenaya spinosa, a species very similar to T. acuelata [ 35]. Alkaloids, glycosides, steroids, flavonoids, saponins and tannins were identified in the methanolic extracts of Cleome viscosa leaves [36]. Species of the genus Cleome present a wide range of chemical compounds that are an excellent option for the treatment of diseases in humans [37]. Silva et al. [18], when studying four types of organic extracts of leaves and roots of T. spinosa, found a broad-spectrum of antimicrobial activity in all analysed extracts and related the activity to the presence of the evaluated phytochemicals, especially flavonoids, terpenoids and saponins. Alkaloids, phenolic compounds and tannins were quantified using standard procedures. T. aculeata stem presented contents of alkaloids higher than the root. Alkaloids are bioactive nitrogen-containing compounds that occur naturally in plants and have been studied by numerous research groups due to the wide range of pharmaceutical activities reported in various plants, such as analgesic, muscle relaxant, antioxidant. However, they can be toxic in large amounts [38,39].
SF showed the highest content of phenolic compounds (312.80 ± 2.77 mg AGE g-1), followed by RF (248.80 ± 2.04 mg ATE g-1), which allows us to verify that the processing of the aqueous extracts to remove water-soluble compounds was effective, resulting in an increase in the levels of phenolic compounds when compared to those of the respective extracts (Table 3). However, the tannin levels did not show significant differences between all analyzed samples, ranging from 43.96 to 44.55 mg ATE g-1 (Table 3). Tannins are polyphenolic compounds known for their high molecular weight and, although multiple biological properties are attributed to them, they have been more extensively studied for their in vitro antioxidant and antimicrobial activities [40].
The phenolic compounds are a group of compounds naturally present in most plants, and these are characterized by having one or more aromatic rings containing one or more hydroxyl groups. This class of bioactive compound has been shown to exhibit, for example, antioxidant, antidiabetic, anti-inflammatory, antiallergic, antiviral, anticancer and antimicrobial activities [41,42]. In the species of the genus Tarenaya, promising antioxidant activity results were found by Bezerra et al. [43], its activity justified by the presence of phenolic compounds present in the aqueous and ethanolic extracts of T. spinosa leaves. In another study, carried out by Silva et al. [38], organic extracts of stems and roots were evaluated, and their antimicrobial activity justified by some metabolites, including flavonoids, a specific class of phenolic compounds that have already been strongly indicated to have antimicrobial activity.
The best results of the methanolic fractions in the analysis performed in relation of the extract can be attributed to the use of Ambertlite XAD-2 resin. This resin technique promotes a concentration of compounds in the fraction [44]. In this study this technique proved to be efficient to increase the levels of phenolic compounds in fractions obtained of the aqueous extracts of T. aculeata. This result corroborates studies in the literature that establish that this technique has been shown to be efficient for the sample preparation, isolation and purification of these constituents [45].
Antioxidant activity has already been observed for Tarenaya species. Bezerra et al. [43] evaluated the antioxidant activity of aqueous and ethanolic extracts of T. spinosa leaves, with IC50 of 377.7 and 445.8 μg mL-1 (Table 4), respectively and attributed this property to the phenolic compounds present in the extracts.
All extracts and fractions showed UVA and UVB absorption, indicating that both extracts and fractions of T. aculeata species are promising as a UV filter (Figure 1). The presence of chemical compounds such as flavonoids, alkaloids and phenolic compounds are associated with the ability of plant extracts to absorb UV radiation in several studies [46]. Flavonoids are polyphenols containing two aromatic rings that present two absorption ranges in the UV region, specifically between 240 and 280 nm and between 300 and 550 nm [47,48].
The resolution RDC No. 30 of June 1, 2012 by ANVISA determines that the photoprotectors must have a minimum SPF of six and a critical wavelength greater than 370 nm for commercial use and that the products must be classified into different categories, according to the calculated SPF value, which can be low (6.0-14.9), medium (15.0-29.9) and high (30.0-50.0) protection, in addition to very high solar protection (greater than 50.0 and less than 100). However, to be considered as a multifunctional product, it needs to have a minimum SPF of two [25]. Among all the extracts and fractions tested, the RF presented the highest SPF value (17.73), followed by the SF (16.58). However, the SE (2.39) can be used in multifunctional formulations, (SPF greater than two) and being classified as low protection products (SPF between 6.0 and 14.9), indicated for skins with little sensitivity to sunburn (Table 4).
The calculation of the critical wavelength allows associating how much protection the sample has against UVA radiation, since the wavelength calculation takes into account the area of the graph relative to 90% of the spectrum area, between 290 and 400 nm [26,49]. The ANVISA requires the minimum value for its use as a sunscreen and therefore, the RE did not present the minimum wavelength value necessary for its use as a photoprotective product [25]. However, the fractions can be considered for future studies related to incorporations in photoprotection as well as the SE products, for multifunctional products.
Thus, combined with the fact that both extracts and fractions absorb UV radiation, and the presence of phenolic compounds and alkaloids that may explain the absorption values found, the SPF values and the critical wavelength evaluated, we can conclude that RF and SF are promising for use in photoprotective formulations in order to achieve a broad spectrum of protection against ultraviolet radiation, and the SE in multifunctional formulations.
The SE and RE showed moderate antibacterial activity (ranging from 125 to 500 μg mL-1), while the SF and RF showed good to moderate activities (MIC ranging from 62.5 to 250 μg mL-1). The antibacterial activities were considered good against the Gran-negative bacteria Pseudomonas aeruginosa, (MIC: 31.25 for the SF and 31.25 for the RF), Escherichia coli (MIC: 62.5, for both the SF and RF) and the Gran-positive bacterium Staphylococcus aureus (MIC: 62.5, for both the SF and RF) (Table 5).
The presence of secondary metabolites found in our studies may justify the results of the antibacterial activity obtained in our study. Phenolic compounds and extracts rich in such secondary metabolites can be excellent inhibitors of pathogenic and spoilage bacteria of food origin [50], alkaloids have an underlying structure, which is capable of enabling the development of antibiotics with a wide range of action [51] and tannins are mainly present in the stems of trees and plants, acting in defense against pathogens, reason that may justify the large number of studies related to its antimicrobial activity [40].
Previous studies have already reported antimicrobial activity for species of the Tarenaya genus. The results obtained for the analysis of antimicrobial activity corroborated those obtained by Nascimento et. al. [52], in which the ethanolic extracts of T. aculeata showed antimicrobial activity against bacteria and fungi. In addition, aqueous extract of T. spinosa leaves showed a MIC value of 512 μg mL-1 against Staphylococcus aureus, indicating relevant antibacterial activity [18].
The hydroalcoholic extract of T. aculeta leaves has already been evaluated in another study involving A. salina, however, it was classified as highly toxic [53]. In another study involving another species of the same genus, T spinosa showed moderate toxicity (LC50: 150 μg·mL-1) for hydroalcoholic root extracts [54]. This corroborates the results obtained in our study, presenting the same classification range as the stem fraction (moderate toxicity).
Despite being classified as low to medium toxicity, the extracts/ fractions SE, SF and RF showed antitumor potential. This is due to the fact that some studies already present the A. salina test as an excellent indicator of biological activity. The extract is considered active when it presents LC50 to 1000 μg mL-1 [55]. The genus Tarenaya does not present studies related to antitumor activity so far, however, the Cleomaceae family stands out for presenting some studies in this regard [34,56]. Hence, more robust studies with the purpose of evaluating the antitumor properties of these strata can be considered in future studies.
The extracts and fractions showed promising antimicrobial agents. In addition, associated with the SPF values found, it can serve as an indication of a multifunctional product formulated from fractions and the SE (except for the RE, with a SPF less than two). In the cosmetics industry, formulations with antimicrobial activity can significantly decrease the use of synthetic preservatives, resulting in a product with less toxic effects and greater stability [46,57].
Conclusions
T. aculeata can biosynthesize alkaloids, phenolic compounds, flavonoids, glycosides, tannins and saponins, and extracts and fractions of stems and roots of this plant were found to have photoprotective, antioxidant and antibacterial potentials. The best photoprotection results were attributed to the SF and RF, while the SE can be indicated as a multifunctional product. The SF and RF showed the best results for antioxidant and antibacterial potentials, confirming that the use of polymeric adsorbent resins such as XAD-2 for the elimination of water-soluble compounds is an efficient strategy to access enriched fractions in bioactive natural products. The RE was considered non-toxic, while SE and SF were of low toxicity and RF was considered of moderate toxicity.
In addition to its low toxicity, the SE, because of its biological profile, also stood out in this research as a promising sample for the development of multifunctional products. However, a detailed chemical characterization and comprehensive toxicological studies will still be needed in order to better understand the medicinal potential of T. aculeata decoctions, as well as whether there are risks when consuming them.