Introduction
Soybean (Glycine max (L.) Merrill) production has dramatically increased in Argentina over the last decades (Pengue & Rodriguez, 2018), with a planted area that amounts to more than 17 million hectares (Ministerio de Agricultura, Ganadería y Pesca, 2019). Defoliator caterpillars, particularly the commonly known “sunflower looper” Rachiplusia nu (Guenêe) (Lepidoptera: Noctuidae), are among the most widespread pests affecting soybean due to their frequency and population levels (Boito et al., 2013).
The natural enemies of insect pests include various predators and parasitoids. Predators comprise spiders (Arachnida: Araneidae), damsel bugs Nabis sp. (Hemiptera: Nabidae) and Podisus sp. (Hemiptera: Pentatomidae), and the beneficial ladybird beetle Eriopis connexa (Germar) (Coleoptera: Coccinellidae), all of which feed on the eggs and tiny larvae of R. nu. Parasitoids include different species of Microhymenoptera and Diptera. The former may be parasite eggs (members of the families Trichogrammatidae, Mymaridae, and Encyrtidae) or larvae (Braconidae and Ichneumonidae) (Hymenoptera: Ichneumonoidea), and Tachinidae (Diptera) members (Freitas Bueno et al., 2012).
In Argentina, the use of insecticides and herbicides in extensive and intensive farming for weed and insect control increased from 73 million kg in 1995 to 310 million kg in 2016 (Etchegoyen & Stimbaum, 2018). This may negatively impact non-target organisms such as soil nutrient recyclers, pollinators, and pest enemies, resulting in biodiversity loss (Ratnieks et al., 2018; Sarandón & Flores, 2014). The side effects of insecticides on pest enemies may include lethal (immediately after application) and sublethal consequences (residual exposure during foraging, oviposition, and feeding) (Arcila-Moreno, 2020; Sarandón & Flores, 2014; Stark & Banks, 2003).
The high mortality induced by broad-spectrum neurotoxic insecticides on beneficial entomofauna has been widely investigated (Fernandes et al., 2008; Fogel et al., 2016; Lemes Fernandes et al., 2010; Schneider et al., 2006). Different studies have reported lethal and sublethal effects after exposure to insecticides with novel mechanisms (selective insecticides) under laboratory conditions (De França et al., 2017; Desneux et al., 2007; Fogel et al., 2016; Lemes Fernandes et al., 2010; Rimoldi et al., 2012; Schneider et al., 2008; Stark et al., 2007). Sublethal effects on natural enemies are divided into two groups: i) physiological (changes in development, decreased longevity, decreased fecundity and sex ratio) (Desneux et al., 2007), and ii) behavioral (changes in individual mobility, the ability to search for preys or hosts, feeding, and oviposition) (De França et al., 2017).
Chlorantraniliprole (Rynaxypyr®, DPX-E2Y45) belongs to a newly introduced class of anthranilic diamide insecticides with a novel mode of action related to activating the arthropod muscular system by interfering with ryanodine receptor binding and causing uncontrolled muscle calcium release. The insecticide affects all larval stages of Lepidoptera, and residues remain for about 21 days on crops (Astor et al., 2009).
This study aimed to determine the effect of chlorantraniliprole applied under field conditions on beneficial arthropods associated with R. nu in soybean crops located in El Triunfo, Lincoln, Buenos Aires, Argentina.
Methods
The study was conducted on crops of large soybean seeds (Glycine max) of the ACA 3939 GR group III variety during the 2014-2015 crop season in El Triunfo (35º 05´ S and 61º 31´ O), Lincoln, province of Buenos Aires, Argentina. The study included two treatment groups: untreated (T1, control; 5 ha) and chlorantraniliprole-treated (T2; 5 ha). The plant-to-plant distance was 35 centimeters, with a 70 meters buffer zone distance between T1 and T2 to mitigate the effect of spray drift. Each sampling unit was divided into four blocks with three sampling points each. The first sampling point was randomly selected for each block, and the remaining points were determined over a zig-zag transect every 30 m. The samples were taken on both treatments for 14 weeks during soybean growth stages (from V2 to V16-R7). A total of twelve samples were collected weekly per treatment (n = 24). Then, the vertical beat sheet (one- meter width) method for sampling insect populations in soybean fields was used (Gamundi, 1995). At each point, R. nu larvae were collected, placed in labeled containers, and taken to the laboratory, where they were individually placed in Petri dishes to continue their growth. They were fed with fresh soybean leaves and maintained in a climate-controlled chamber at 25 °C ± 2 °C, 60% relative humidity, and 16:8 photoperiod to detect parasitic larvae (Avalos et al., 2004). At the same time, predator arthropods associated with soybean crop pests were also recorded at each sampling point (Massaro, 2005).
Chlorantraniliprole (30 cm./ha) was applied on January 20, 2015, at soybean growth stages V10- R2 on T2 once the R. nu population reached the economic injury level (EIL), which establishes different soybean prices for larvae > 1.5 cm, according to the phenological stage of crops and water regimes (Iannone, 2012). The insecticide was applied with conventional self-propelled equipment (18 m spraying width, hollow cone nozzles, 120 l/ha water volume). No insecticide was applied to T1.
Statistical analysis
Data were analyzed with the Generalized Linear Mixed Model (GLMM) for repeated measures. Differences between means were analyzed with Tukey’s test (p < 0.05) using InfoStat, version 2019 (Di Rienzo et al., 2011). The effect of chlorantraniliprole on the population richness and abundance of beneficial insects was determined with the Shannon-Wiener diversity (Chao et al., 2005) and Berger-Parker dominance (Moreno, 2001) indices, respectively.
Results and Discussion
Beneficial predators can be found in many orders and families of Insecta and Arachnida. Insect predators most frequently belong to species from the orders Hemiptera (Anthocoridae, Pentatomidae, and Reduviidae), Coleoptera (Carabidae, Coccinellidae, and Staphylinidae), Chrysopidae (Cecidomyidae and Syrphidae) and Hymenoptera (Formicidae). In Arachnida, beneficial species include the orders Araneae and Acari. In the case of parasitoids, they mainly belong to the orders Hymenoptera and Diptera (Nicholls Estrada, 2008).
For several years, studies on insecticide toxicity in beneficial arthropods were focused on acute toxicity, avoiding sublethal effects on physiology, demography, and life tables to avoid long-term population effects (Amarasekare et al., 2016).
We found significant differences (GLMM; p < 0.05) in population abundance for some natural enemies (non-target organisms), such as predators of the orders Araneae and Diptera: Syrphidae, and parasitoids of the genus Copidosoma (Ashmead) (Hymenoptera: Encyrtidae) before and after insecticide application (Table 1).
Table 1. Population abundance of beneficial arthropods in both study groups
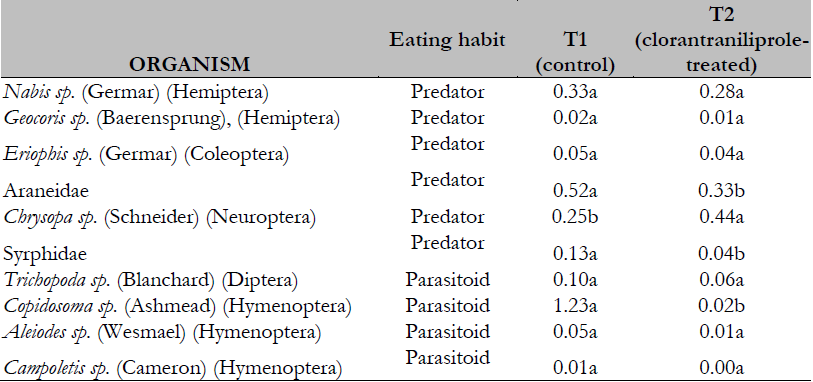
*Different letters in the same row indicate significant differences (p < 0.05).
Source: Own elaboration.
The Shannon-Wiener diversity index (H´) showed similar values in T1 during the cultivation period. After chlorantraniliprole application in T2, diversity values decreased by 20% (Table 2). Even though chlorantraniliprole did not affect the beneficial fauna richness of the crop field evaluated, the loss of species abundance that may result from insecticides harms the system’s biodiversity. The possible risks of applying new-generation insecticides on beneficial arthropods (parasitoids and predators of pest insects) may have direct and indirect effects. Direct effects refer to adverse effects caused by exposure of non-target organisms to the active component of the insecticide. In contrast, indirect effects on parasitoids may be due to changes in host quality (even by interrupting its cycle, leading to host death) (Morales et al., 2004) and the elimination of prey, with the consequent diversity loss in terms of richness and abundance of the agroecosystem (Altieri & Nicholls, 2000). Differences in the Shannon-Wiener index (H´) before and after chlorantraniliprole application in T2 showed the possible biodiversity loss after applying insecticides, including new-generation ones.
Table 2. Shannon-Wiener diversity index (H´) and Berger-Parker index (D)
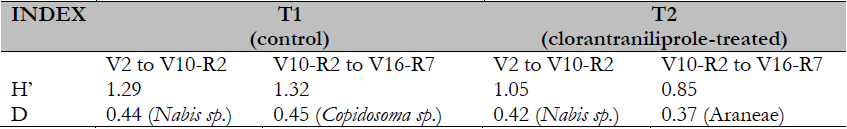
Note. VEFirst trifoliate leaf is fully expanded. V10-R2 (vegetative 10, reproductive 2). V16-R7 (vegetative 16, reproductive 7). Fehr and Caviness Scale (1971).
Source: Own elaboration.
The Berger-Parker dominance index exhibited changes in the number of beneficial insect species predominating in the system after applying chlorantraniliprole. Before application, Nabis species predominated in T1 and T2. After applying the insecticide (January 20), the most representative species were microhymenopteran parasitoid Copidosoma sp. in T1 and generalist predator taxon Araneae in T2 (Table 2). This could result in changes in beneficial individual patterns in the agroecosystem, favoring the species or groups of species less affected by these insecticides, as evidenced by the Berger-Parker dominance index.
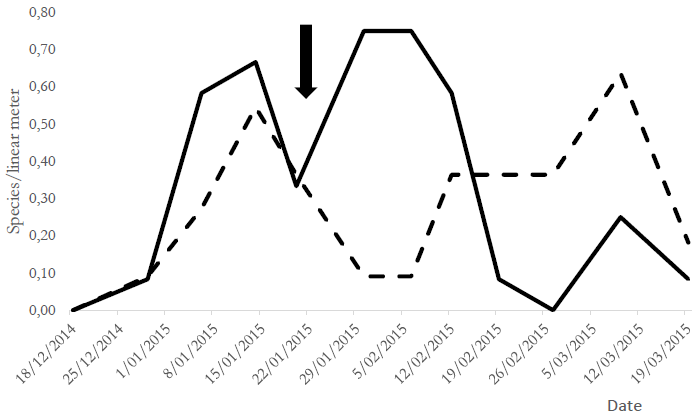
The assessment of population fluctuation of the species evaluated before and after chlorantraniliprole application showed that the population abundance of R. nu in T1 decreased after seven days and reached values below EIL after 15 days. The population abundance of Copidosoma sp. increased concomitantly with R. nu decrease in T1, while it decreased significantly in T2 after applying the insecticide (Figure 1).
Studies evaluating lethal and sublethal effects on natural enemies after field exposure to selective insecticides are scarce (Biondi et al., 2013), despite laboratory assays alerting the need to confirm such impact in field studies (Turchen et al., 2016). Knowing the potential effect of new- generation insecticides on natural enemies is relevant to advance in the integration of chemical and biological control measures (Ramos et al., 2018).
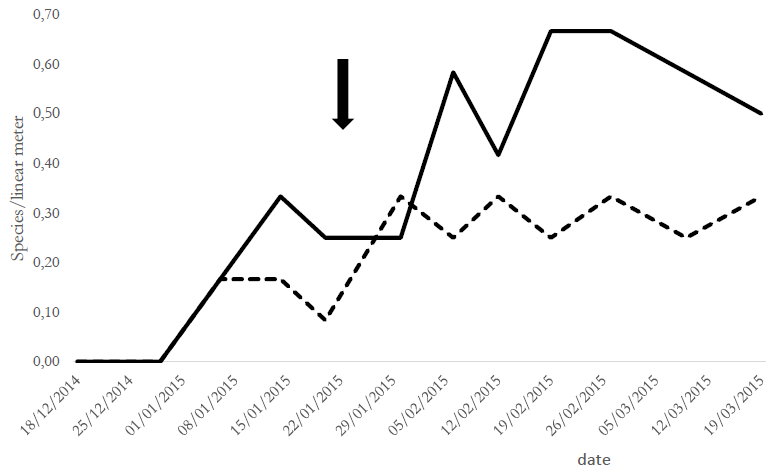
The present study observed a significant decrease in the Copidosoma sp. population after applying chlorantraniliprole. The population fluctuation of parasitoids and predators taken together before and after chlorantraniliprole application (analyzed by GLMM) indicated that parasitoids decreased significantly compared with the control group (. > 0.05) (Figure 2).
The Berger-Parker index showed the dominance of the order Araneae in T2 after applying chlorantraniliprole. However, the growth curve of the arachnid population was affected as compared with the untreated control, suggesting the taxon sensitivity to anthranilic diamides. The GLMM analysis of the population fluctuation of predators by species after applying chlorantraniliprole showed that the increase in the spider population in T2 was not as marked as in T1 (p > 0.05) (Figure 3).
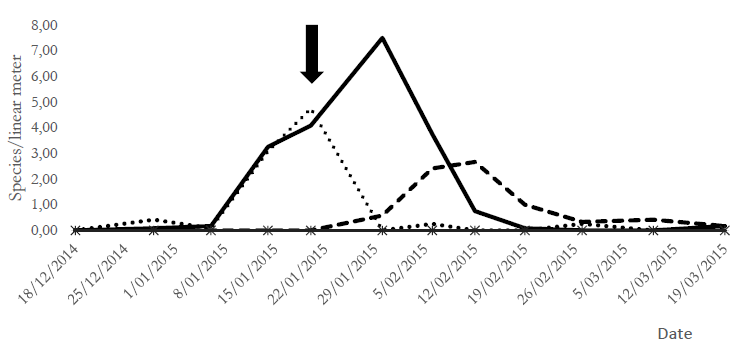
Source: Own elaboration.
Figure 1. Population fluctuation of Rachiplusia nu larvae and parasitoid Copidosoma sp. in both treatment groups.References: Rachiplusia nu = T1 (control), T2 (chlorantraniliprole-treated); Copidosoma = T1 (control), T2 (chlorantraniliprole-treated). The arrow indicates insecticide application in T2 during soybean growth stages V10-R2 (vegetative 10 and reproductive 2).
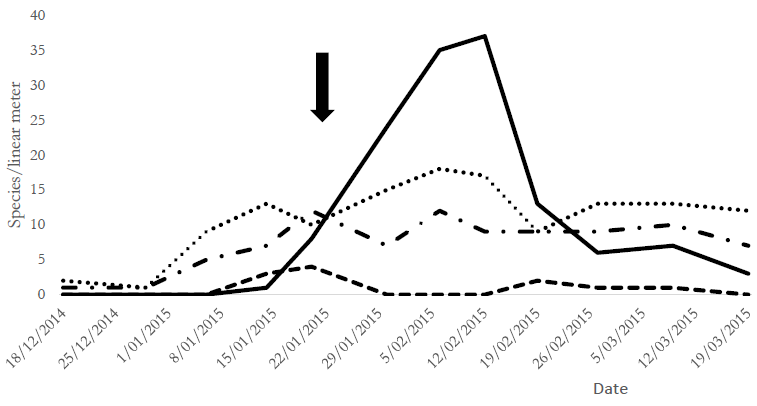
Source: Own elaboration.
Figure 2. Population fluctuation in parasitoid and predator populations before and after chlorantraniliprole application.References: predators = T1 (control), T2 (chlorantraniliprole-treated) *; parasitoids = T1 (control), T2 (chlorantraniliprole- treated) . The arrow indicates insecticide application in T2 during soybean growth stages V10-R2 (vegetative 10 and reproductive 2).
Source: Own elaboration.
Figure 3. Population fluctuation of Araneae predators before and after chlorantraniliprole application.References: T1 (control), T2 (chlorantraniliprole- treated). The arrow indicates insecticide application in T2 during soybean growth stages V10-R2 (vegetative 10 and reproductive 2).
Benamú et al. (2013) reported direct lethal effects on spider mortality and sublethal effects on web-building capacity, mating behavior, fecundity, and fertility of eggs after applying spinosad, a natural neurotoxic insecticide. These effects could contribute to the lower population growth rate of the studied spiders. In this sense, Baloriani et al. (2010) agreed on the greater population abundance of spiders in greenhouses managed with reduced chemical inputs, characterized by decreased use of agrochemicals, higher crop diversity, and spontaneous vegetation, providing a natural environment, shelter, and food for spiders, as compared with conventional management. Benamú et al. (2010) observed that only 20% of spiders exposed to glyphosate could build their webs normally, while eating habits were affected due to the difficulty of capturing their prey.
The Nabis sp. population fluctuation was significantly affected by GLMM (p > 0.05). Its population decreased immediately in T2 after applying the insecticide compared with T1 (Figure 4). Nevertheless, around the eighth week after application, the species population level in the chlorantraniliprole-treated field crop recovered, possibly once the residual effect of the anthranilic diamide disappeared. In agreement with these data, Bonetti (2012) showed that chlorantraniliprole was not selective for Nabis sp. with the field doses applied. Laboratory assays by Viñuela et al. (1998) showed the lethal effect of topical applications of spinosad on the hemipteran predator Podisus maculiventris (Say), which led to death during the nymphal stage. After applying azadirachtin, an insect growth regulator (IGR), sublethal effects resulted in adult malformations. Both are considered selective insecticides for beneficial fauna. Naranjo and Akey (2005) reported that 17 taxa of predator arthropods were affected negatively by the IGR application.
Source: Own elaboration.
Figure 4. Population fluctuation of hemipteran predator Nabis sp. before and after chlorantraniliprole application.References: T1 (control), T2 (chlorantraniliprole-treated). The arrow indicates insecticide application in T2 during soybean growth stages V10-R2 (vegetative 10 and reproductive 2).
In future studies, we will evaluate the effect of chlorantraniliprole on other predators or parasitoids and continue studying lethal and sublethal effects on beneficial fauna caused by other insecticides with Modern Action Modes through field essays.
Conclusion
The application of chlorantraniliprole under field conditions decreased the diversity of natural enemies associated with soybean cultivation. Parasitoids of the order Hymenoptera and predators of the taxon Araneae were among the most affected organisms. Research so far has contributed to the knowledge of the effects of new-generation insecticides on beneficial fauna associated with soybean crops. Future research on selective insecticides with other modes of action would be needed.
Authors’ contributions
Carolina Sgarbi: registration of information in the field, taxonomic identification of specimens, construction of databases, analysis of information and preparation of manuscript; Cecilia Beatriz Margaría: design of methodologies, supervision of activities, taxonomic identification of specimens, analysis of information and preparation of manuscript; Elisabet Mónica Ricci: preparation of project for access to economic resources, design of methodologies, supervision of activities, analysis of information and preparation of manuscript.
Ethical implications
The present article has the endorsement 09-2021 filed on November 23, 2021, from the research ethics committee of the Universidad Nacional Noroeste de la Provincia de Buenos Aires. Consent was also obtained from the collaborators to use the information provided in the documentation of the process presented in the article.















