INTRODUCTION
Cervical cancer (CC) is a serious public health problem [1]. Despite the advances in diagnostic techniques and the inclusion of the Pap smear as a screening [2] and control measure for CC, more than 530 000 new cases are estimated annually in women worldwide [3]. In Brazil, this type of cancer is the fourth leading cause of cancer-related mortality amongst Brazilian women [4] According to the National Cancer Institute, for the biennium 2018-2019 there will be an estimated 16 370 cases of CC per 100 000 women [5].
Currently, more than 200 types of human papillomavirus (HPV) have already been cataloged [6,7], there being 15 subtypes with oncogenic power [8,9], which cause 80% of low-grade squamous intraepithelial lesions (LSIL), and 90% of high-grade squamous intraepithelial lesions (HSIL) [10]. These subtypes are present in 99% of cases of CC [11,12], with the HPV genotypes 16 and 18 being the most prevalent worldwide [13]. About 90% of immunocompetent women infected with some oncogenic HPV subtype have a spontaneous immune response, which plays a key role in eliminating the virus within three and a half years, however, 1% of these women progress to the development of invasive CC [14].
In recent years, different studies have demonstrated the important role of the immune system in controlling tumor growth and progression [15]. It is known that the uterine cervix is a favorable organ for HPV infections, promoting an innate and adaptive immune response [16]. This cell-mediated response is fundamental for the control of infections, progression of cervical lesions and malignancy HPV-associated disease [14,17].
In recent years, different studies have demonstrated the important role of the immune system in controlling tumor growth and progression [15]. This cell-mediated response is fundamental to avoid squamous intraepithelial lesions (SIL) and malignant HPV-associated disease [14,16]. However, a virus with an imbalance of the host immune response, promotes several escape mechanisms, inhibits the action of antigen-presenting cells and the manipulation of Th1/Th2 polarization [17]. Moreover, when chronic inflammation persists in the cervical microenvironment, immune responses play a critical role during HSIL progression [18]. In other studies, it was observed that inflammation is considered a key point for the development of different cancers, acting on tumor progression, stimulating cell proliferation, invasion, metastasis and angiogenesis [19,20]. This may occur due to suppression at the tumor site, via antitumor and pro-inflammatory response [21,22].
In addition to this, some cytotoxic cytokines are activated, promoting signaling and agglomeration of (CD) 4+ T helper cells. Unlike regulatory T cells, Forkhead box P3 + (FOXp3 +) T cells suppress the immune response of other cells, inhibiting the inflammatory process [23].
In another study, it was also reported that monocytes, eosinophils and dendritic cells (DC) could be observed in biopsies of nasopharyngeal carcinoma [24]. While in rectal cancer, several markers of innate and adaptive immune response have been observed, resulting in cell infiltration [25]. In addition, other cells such as fibroblasts can activate some infiltrating inflammatory cells, favoring the growth of cancer cells and allowing metastatic dissemination [26].
Recently, several studies have shown that the increase of inflammatory cells, such as neutrophils and lymphocytes in the peripheral blood is a good indicator for different cancers, but in situ studies are scarce [27]. As the collection of cytology and biopsy material from the cervix is easy to access, the need to understand the local response is of great relevance.
Therefore, the aim of the present study was to investigate the plasma density of inflammatory infiltrate cells in biopsy specimens of women with or without HPV-induced cervical lesions.
MATERIAL AND METHODS
Ethical approval
The study received approval from the institutional board of the Federal University of Rio Grande do Norte (UFRN) (protocol N.° 526/11).
Subject
For this study, 108 women were recruited in the Cervical Pathology outpatient clinic of a tertiary hospital. All recruited women received detailed information regarding the objective of the study and gave written consent to participate.
Sample collection and Cytology
Histopathological analysis was performed on sections from paraffin blocks of 4 µm thickness and stained with hematoxylin/eosin. All analyzes followed the criteria established according to the Bethesda Classification. These specimens were stratified into four groups: Low-grade squamous intraepithelial lesion (LSIL), High-grade squamous intraepithelial lesion (HSIL), cervical cancer (CC) and negative for intraepithelial lesion and malignancy (NILM). All biopsies were done with hematoxylin and eosin (HE) and analyzes were performed by two independent pathologists.
The morphometric analysis was performed from the identification and quantification of inflammatory cells in ten random fields per sample. The representative images of the stroma of the cervix biopsies were taken at 400X magnification using a digital camera attached to the histomorphometric microscope. Each chosen field has an area of 0.066 mm2. These images of the biopsies samples were captured by a digital system and analyzed using the Leica Qwin Pro V 3.5.1 software, from Leica Microsystems Ltd (Switzerland, Whisker). The morphometric parameters were the number of lymphocytes, plasmocytes, neutrophils and eosinophils. The number of cells per parameter counted in areas of stroma was obtained for each case.
Statistical analysis
The differences between groups were assessed using parametric Anova and Tukey Multiple Comparison Test. Mean and minimum-maximum for each morphometric parameter were obtained. Anova test was used to determine if there were statistically significant differences between the number of cells per field and per mm2 counted in the stroma among NILM, LSIL, HSIL and CC groups. Tests yielding p-values <0.05 were considered significant. We used the Graphpad Prism 5.0.
RESULTS
Demographic and clinic characteristics of the study participants by the histological group did not show statistical differences between age (years), age at first intercourse, number of lifetime sexual partners, ethnicity, education level, contraceptive method, alcohol use and smoking habit (table 1).
Table 1 Demographic and clinicopathological characteristics observed in patients with cervical lesion and cancer.
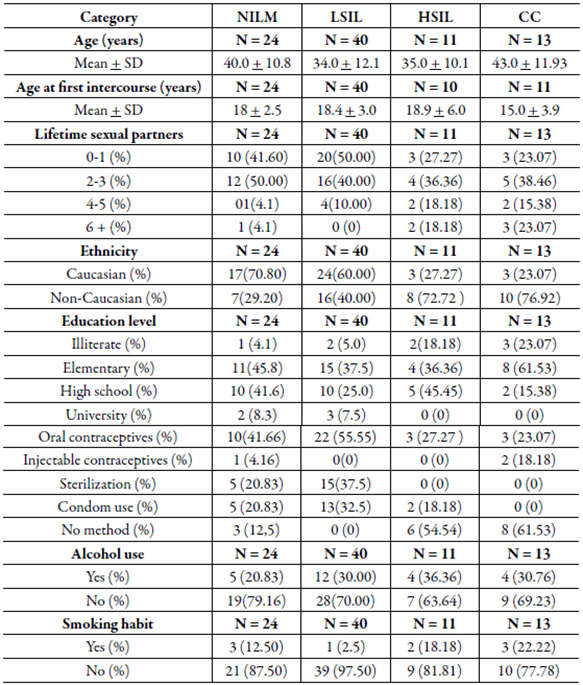
NILM: negative for intraepithelial lesion malignancy; LSIL: Squamous intraepithelial lesion; HSIL: High-grade squamous intraepithelial lesion; CC: cervical cancer.
In the analysis by cells/mm2 density, the mean of lymphocytes was significantly higher in the CC group when compared with LSIL, HSIL and NILM groups (table 2 and figure 1a). The analysis demonstrated a significant increase in the number of lymphocytes among the CC group. However, no difference between the number of neutrophil cells, plasmocytes cells and eosinophils was observed among the groups figure 1b, 1c and 1d).
Table 2 Quantitative distribution (mean, minimum and maximum) in the number of inflammatory cells (cells/mm2) in cervical precursor lesions and cancer.
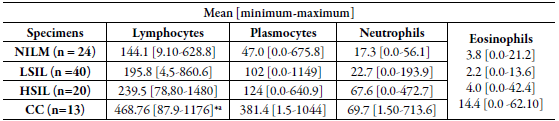
NILM: negative for intraepithelial lesion malignancy; LSIL: Squamous intraepithelial lesion; HSIL: High-grade squamous intraepithelial lesion; CC: cervical cancer.
*a Tests yielding p-values <0.05 were considered significant.
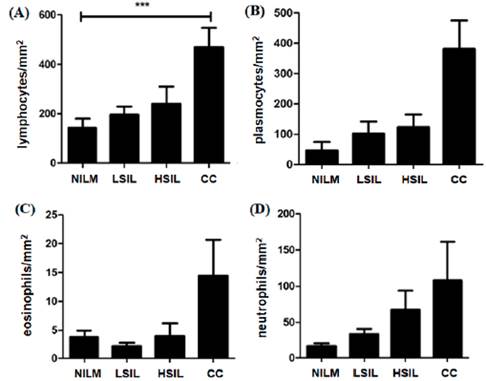
Figure 1 Significant increase in mean number density (cells/mm2) of lymphocytes were found in CC patients compared to NILM patients (A). Comparing the average number (cells/mm2) of plasmocytes (B), eosinophils (C), neutrophils (D) and cells of LSIL. HSIL and CC patients with NILM showed no statistical significance. (A)*** Tests yielding p-values <0.05 were considered significant. NILM: negative for intraepithelial lesion malignancy; LSIL: squamous intraepithelial lesion; HSIL: High-grade squamous intraepithelial lesion; CC: cervical cancer.
DISCUSSION
During this study, we histomorphometrically analyzed the density of immunoinflammatory cells in cervical microenvironment of women with different degrees of SIL and CC. We verified that CC biopsy specimens had a significant increase in the number of lymphocytes compared with NILM, LSIL and HSIL.
The immune response is extremely important in restraining these neoplastic cells, and failures in this system may lead to cancer progression [14]. In this case, the presence of cytotoxic lymphocytes is beneficial to the patient concerning HPV virus elimination, providing protection against tumor progression, since the specialty of this cell type is the immune response against viruses [14,17].
However, other studies have found that the presence of tumor-infiltrating lymphocytes (TIL) in the uterine cervix induced the migration of these cells into the tumoral environment and is directly proportional to the progression of the uterine lesions, proving to be a type of ineffective response, which enables the neoplastic progression of CC [28].
Previously, a cohort study demonstrated an association between the decreased number of lymphocytes and the increased risk of relapse in cervical cancer, confirming the importance of the immune system in immunosurveillance to prevent progression of the intraepithelial lesion [ 26].
Recently, a greater presence ofCD4+ and CD8+ lymphocytes was detected in the tumor tissue of the uterine cervix, when compared to those found in the peripheral blood [29].
In another study, it was analyzed that the presence of the Treg cells in the TIL can cancel the cytotoxicity of the NK cells [30]. Evidence indicates that B cells play a significant role in antitumor immune responses. Although only low numbers of B cells infíltrate premalignant lesions, these cells may exert a distant effect on oncogenesis by secreting antibodies, which are deposited at the tumor site in the form of immune complexes [31]. Researchers found lower amounts of CD20 in patients with SIL and in intratumoral tissue; however, it is present at increased levels in the peritumoral stroma. B cells do not seem to act directly on the tumor tissue, but at a distance, through the secretion of antibodies [32].
In another study, there was an increase in eosinophil infiltrates and their density increased according to the progression of cervical cancer [33]. The presence of eosinophils is reported as a prognostic biomarker in CC [34,35]. In our results, despite the absence of statistical significance, we found an increase in cancer samples compared to the LSIL. Our findings corroborate with those of Spiegel et al. [ 36], which associated the presence of eosinophils with the capability to invade CC, in a retrospective study. Van Driel et al. [ 37] also demonstrated an increased influx of eosinophils in cancer samples and correlated this with a loss of effective immunity. In a later study, the presence of eosinophils correlated tumors with local secretion of IL-4, a Th2 profile of interleukin which is typically anti-inflammatory [38]. In the double-labeling immunohistochemistry, it was shown that cells expressing IL-4 were also CD3+. These findings suggest that the presence of an eosinophilic infiltrate is mediated by IL-4 and is a result of a type 2 response mediated by CD3+ T lymphocytes.
This study has some limitations; firstly, it was a cross-sectional study with a small sample size. Secondly, we were unable to adjust for certain critical confounders such as systemic infection or inflammation, and the number of lymphocytes, plasmocytes, neutrophils and eosinophils may have been affected by the presence of other systemic diseases.
The study was designed with a collection of slides that were in the Department of Pathology/UFRN, the images were captured in the Laica histomorphological microscope, but due to time, we had losses of the images saved on the computer. At the pre-sent moment we were unable to recover the images referring to this study.
CONCLUSIÓN
Cervical cancer biopsy specimens had significantly more lymphocytes / mm2 than NILM, LSIL and HSIL. The role of tumor-infiltrating lymphocytes is not yet well established. Studies demonstrate that CD3 + lymphocyte infiltrates correlate with the presence of metastases at diagnosis, while CD4 + and CD20 + lymphocytes are related to the characteristics of better prognosis in CC. Some studies emphasize the importance of infiltration of FoxP3 + lymphocytes (regulatory T) and their correlation with characteristics of tumor aggressiveness, tumor size and presence or absence of invasion, but further studies in patients with CC and SIL are important to better understand the role of this immunomodulation represented by tumor-infiltrating lymphocytes.














