Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Ciencias Pecuarias
Print version ISSN 0120-0690On-line version ISSN 2256-2958
Rev Colom Cienc Pecua vol.24 no.2 Medellín Apr./June 2011
Relationship among damaged chromatin, motility and viability in cryopreserved spermatozoa from Brahman bulls¤
Relación entre la alteración de la cromatina, movilidad y viabilidad en espermatozoides criopreservados de toros Brahman
Relação entre a alteração da cromatina, motilidade e viabilidade dos espermaotozoides criopreservados de touros Brahman
Héctor Nava-Trujillo 1,5*, MV, Esp; Armando Quintero-Moreno 1,2, MV, MS, PhD; Geovanny Finol-Parra 3, MV, MS; Gabriela Carruyo 4, MV, MS, PhD; Venuslira Vilchez-Siu 4, MV; Carla Osorio-Meléndez 1,2, MV; Jorge Rubio-Guillén 1,2, MV, MS; Robert Valeris-Chacín 4, MV, MS.
1 Laboratorio de Andrología,
2Unidad de Investigación en Producción Animal (UNIPA),
3Departamento de Biología Animal,
4Departamento de Enfermedades Transmisibles. Facultad de Ciencias Veterinarias, Universidad del Zulia (LUZ), Maracaibo 4005-A, Estado Zulia-Venezuela. Apto. Postal 15252.
5Fundo La Rosita, El Guayabo, Estado Zulia-Venezuela.
(Recibido: 20 abril, 2010; aceptado: 26 abril, 2011)
¤ To cite this article: Nava-Trujillo H, Quintero-Moreno A, Finol-Parra G, Carruyo G, Vilchez-Siu V, Osorio-Meléndez C, Rubio-Guillén J, Valeris-Chacín R. Relationship among damaged chromatin, motility and viability in cryopreserved spermatozoa from Brahman bulls. Rev Colomb Cienc Pecu 2011; 24:116-122.
* Corresponding author: Héctor Nava-Trujillo. Facultad de Ciencias Veterinarias, Apto. Postal 15252. Universidad del Zulia (LUZ), Maracaibo 4005-A, Estado Zulia-Venezuela. E-mail: hectornava00@hotmail.com.
Summary
The aim of this study was to determine the percentage of sperm with damaged chromatic measure with toluidine blue stain and it´s relationship with motility and viability in criopreserverd semen from Brahman bulls. Three ejaculates from six Brahman bulls were used. Immediately after thawing, sperms were stained with toluidine blue to establish chromatin integrity (sperms with normal chromatin were light blue or green while sperms with damaged chromatin were dark blue or violet). Sperms were also stained with eosin-nigrosin to determine viability (live sperms were unstained while dead sperms were pink). Motility was measured under light microscope. Effects of bull, ejaculate, and the interaction between variables were assessed. The percentage of live sperms was 50.02 ( ± 14.13%). The mean motility was 33.88 (± 12.43%), while the percentage of sperms with damaged chromatin was 4.17 ( ± 2.96%). Viability was positively correlated with motility (r=0.77217, p=0.0002), and negatively correlated with damaged chromatin sperms (r= -0.43104, p=0.0087). Motility percentage was negatively correlated with the percentage of sperms with damaged chromatin (r=-0.48337, p=0.0421). In conclusion, cryopreserved semen of Brahman bulls presented a low level of chromatin damage, and this trait was negatively correlated with sperm motility and viability.
Key words: brahman bulls, cryopreservation, sperm chromatin, toluidine blue stain.
Resumen
El objetivo del presente trabajo fue determinar el porcentaje de espermatozoides con cromatina dañada medida con la tinción de azul de toluidina, y su relación con la motilidad y la vitalidad del semen criopreservado de toros Brahma. Para ello, se utilizó semen de tres eyaculados de seis toros Brahman, el cual una vez descongelado se procedió a teñir con azul de toluidina para determinar la integridad de la cromatina (espermatozoides con cromatina normal teñidos de azul o verde claro; espermatozoides con cromatina anormal teñidos de azul oscuro o violeta), también se tiñeron con eosinanigrosina para determinar la viabilidad (espermatozoides vivos permanecen blancos; espermatozoides muertos se tiñen de rosado) y se estimó la motilidad espermática mediante microscopía óptica. Se evidenciaron las diferencias en todos los parámetros evaluados debidas al efecto toro y al eyaculado, así como a la interacción entre estas dos variables. El porcentaje de espermatozoides vivos fue de 50.02 ± 14.13% y la motilidad espermática promedió un 33.88 ± 12.43%, mientras que el porcentaje de espermatozoides con cromatina dañada fue de 4.17 ± 2.96%. El porcentaje de espermatozoides vivos se correlacionó positivamente con la motilidad (r=0.77217, p=0.0002), y negativamente con el porcentaje de espermatozoides con cromatina dañada (r= -0.43104, p=0.0087), mientras que el porcentaje de motilidad se correlacionó negativamente con el porcentaje de espermatozoides con cromatina anormal (r= -0.48337, p=0.0421). En conclusión, el semen criopreservado de toros Brahman presenta un bajo nivel de espermatozoides con daño en la cromatina, lo cual se correlaciona negativamente con la motilidad y la vitalidad espermática.
Palabras clave: criopreservación, cromatina espermática, tinción azul de toluidina, toros brahman.
Resumo
O objectivo deste estudo foi determinar a percentagem de espermatozóides com cromatina danificada, determinada pela coloração com azul de toluidina e sua relação com a viabilidade e a mobilidade do esperma cripreservado de touros Brahman. Para isso, foram utilizados três ejaculados de sêmen de seis touros Brahman, que uma vez descongelado foram coradas com azul de toluidina para determinar a integridade da cromatina (espermatozóides com cromatina normal coloream de azul ou verde; cromatina de espermatozóides con cromatina danificada, coloream de azul escuro ou violeta). Também foram corados com eosina nigrosina para determinar a viabilidade (espermatozóides vivos permanecem brancos e os mortos de cor rosa) e a motilidade espermática foi estimada por microscopia de luz. Foram encontradas diferenças significativas em todos os parâmetros, devido ao efeito de touro e o ejaculado, bem como a interacção entre essas duas variáveis. A percentagem de espermatozóides vivos foi de 50.02 ± 14.13% e motilidade espermática média de 33.88 ± 12.43%, enquanto a percentagem de espermatozóides com cromatina danificada foi de 4.17 ± 2.96%. A percentagem de espermatozóides vivos foi positivamente correlacionada com a motilidade (r=0.77217, p=0.0002) e negativamente com a porcentagem de espermatozóides com cromatina danificada (r = -0.43104, p= 0.0087), enquanto que a percentagem de motilidade correlacionou negativamente com a percentagem de espermatozóides com cromatina danificada (r = -0.48337, p=0.0421). Em conclusão, o sêmen de touros Brahman criopreservados tem um baixo nível de dano da cromatina, que está correlacionada negativamente com a motilidade e a vitalidade do esperma.
Palabras-chave: criopreservação cromtina espermatica, coloração de azul de toluidina, touros.
Introduction
Sperm quality is a very important issue to reach optimal reproductive efficiency in herds and in vitro embryo production systems, because sperm can affect embryo development during the early stages after fertilization (Eid et al., 1994; Hansen, 2002). Sperm DNA composition and structure differs from somatic cells DNA. The DNA in sperm is disposed in chromatin, which is formed by DNA and basic proteins called protamines. The cysteine residues in protamines form disulfide bonds which compact the DNA six times more than in somatic cells. These bonds protect the genetic material from stressing agents such as reactive oxygen species and high temperatures during the transit through the male and female reproductive tract (Evenson et al., 2002; Kosower et al., 1992; Oliva, 2006; Zini et al., 2001).
Along with classical parameters of sperm quality evaluation (Gillan et al., 2008; Januskauskas et al., 2001), chromatin integrity is currently considered a determinant part of sperm quality. In bulls, the level of sperm with damaged chromatin has been negatively correlated with motility and viability (Januskauskas et al., 2003; Kasimanickam et al., 2006; Khalifa et al., 2008). Sperm chromatin integrity has been shown to affect the reproductive potential in several studies (Ballachey et al., 1987; Dobrinski et al., 1994; Madrid-Bury et al., 2005).
Several factors have shown to affect chromatin integrity. There exists ample evidence on the possibility of cryopreservation negatively affecting sperm DNA integrity. However, its precise underlying mechanism is still much debated. A recent study reports that cryopreservation-induced injury to sperm DNA is mediated primarily through oxidative stress rather than apoptosis (Thomson et al., 2009). Semen cryopreservation induces sperm DNA fragmentation, which is comparatively higher in bulls with poor semen quality (Mukhopadhyay et al., 2011).
Techniques such as Sperm Chromatin Structure Assay (SCSA; Ballachey et al., 1987; Bochenek et al., 2001; Charles-Ostermeier et al., 2001; Januskauskas et al., 2001; Januskauskas et al., 2003; Hallap et al., 2005; Madrid-Bury et al., 2005; Waterhouse et al., 2006), Terminal Deoxynucleotidyl Transferase mediated dUTP nick end labelling (TUNEL; Fatehi et al., 2006; Waterhouse et al., 2006), Acridine Orange (AO; Khalifa et al., 2008; Celeghini et al., 2008), and Comet assay (Slowínska et al., 2008; Urrego et al., 2008) have all been used to evaluate sperm chromatin integrity in fresh and thawed semen from bulls. These techniques are expensive and time consuming, which limits their application in research laboratories. Therefore the use of alternative methods to evaluate sperm chromatin integrity during the routine sperm quality evaluation could give more information on this important parameter and the factors affecting the reproductive potential of bulls.
Toluidine Blue (TB) is a nuclear dye used to evaluate sperm chromatin integrity by detecting the absence or rupture of disulfide bonds. Thus, dark stained nuclei indicate altered sperm chromatin. This stain has been used to evaluate sperm chromatin integrity in several species, such as bulls, horses, rabbits, buffaloes and humans (Mello, 1982; Beletti and Mello, 1996; Mello and Beletti, 2002; Beletti and Mello, 2004; Erenpreisa et al., 2003; Beletti et al., 2005; Sardoy et al., 2008; Zúccari et al., 2008). In humans, TB stain showed high correlation with SCSA, TUNEL and AO (Erenpreisa et al., 2003; Erenpreiss et al., 2004); while in rabbits, it showed a high correlation with Feulgen reaction (Beletti and Mello, 2004), validating TB as a technique suitable for studying sperm chromatin integrity. However, most studies published so far were conducted using Bos taurus taurus semen and scarce information has been published about levels of sperm with damaged chromatin and its relationship with another parameters of sperm quality in Bos taurus indicus bulls, especially in Brahman bulls. Therefore, the aim of this study was to determine the percentage of sperm with damaged chromatin measured with toluidine blue stain and its relationship with viability and motility in cryopreserved semen of Brahman bulls.
Materials and methods
Semen samples
Three ejaculates from six Brahman bulls were used. Bulls were located at the VIATECA Artificial Insemination Center in Machiques County, Zulia State, Venezuela. Ejaculates were collected using an artificial vagina. After routine evaluation, only ejaculates that reached at least the minimum quality standards established by the Center for Brahman bulls were processed (concentration: 850x106 sperm/mL; individual progressive motility: 60%). Semen was diluted in two stages to reach a final concentration of ~30x106 motile sperm/mL. After dilution and equilibration at 5 °C, semen was loaded into 0.5 mL straws, which were frozen in liquid nitrogen vapours 4 cm above N surface for 10 min, and then plunged into the liquid nitrogen. Semen extender was prepared with skim milk, egg yolk medium (20%), and glycerol (7%).
Motility and viability in thawed semen
Immediately after straw thawing in a water bath at 37 °C for 30 seconds, motility was estimated under light microscope at x 400 magnification in bright field illumination. Sperm viability was measured with eosin-nigrosin stain (Tamuli and Watson, 1994), mixing 15 μL of semen and 10 μL of stain for 30 seconds. Spermatozoa were smeared onto a pre-warmed glass slide and air dried. The percentage of viable (unstained) sperm was determined by smear observation under light microscope at x 1000 magnification. These analyses were conducted by one technician.
Chromatin integrity in thawed semen
Sperm chromatin integrity was assessed with toluidine blue stain (Agarwal and Said, 2004). Air dried semen smears were fixed in an ethanol:acetic acid (3:1 v/v) solution for 30 minutes and then smears were hydrolysed for 5 minutes in 0.1 N HCl, washed in distilled water, air dried and stained with a 0.05% toluidine blue (pH 4) in McIlvain buffer for 10 minutes (Agarwal and Said, 2004). Smears were observed under a light microscope at x 1000 magnification. Light blue or green sperm were considered as having normal chromatin, whereas dark blue or violet sperm were considered as having damaged chromatin. This analysis was conducted by one technician.
Statistical analysis
At least 200 cells were counted in duplicate for each smear in both eosin-nigrosin and TB. Statistical data analysis was conduected using Statistical Analysis System for Windows, software 8.2 (SAS Inst. Inc.; Cary, NC. USA). Percentages of viability and sperm with normal chromatin integrity were transformed through the square arcsine method to obtain a normal distribution. Analysis of variance was conducted with the General Lineal Model (GLM procedure) and results are shown as means ± SD. Bull and ejaculate effects on viability, motility and percentage of sperm with abnormal chromatin integrity were evaluated. A Spearman's correlation test was used to establish correlation among the measured parameters.
Results
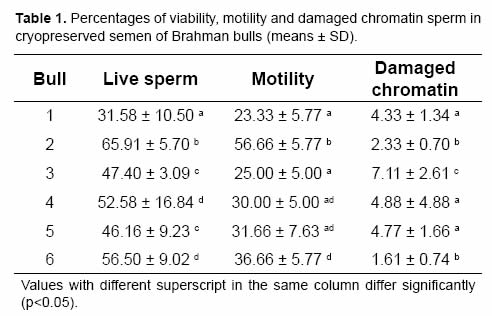
The analysis of variance showed that percentages of viability, motility and sperm with damaged chromatin were significantly affected by bull, ejaculate and the interaction between both variables. Results per bull are shown in Table 1. Percentage of live sperm averaged 50.02 ± 14.13. Motility ranked from 23% to 56%, with a mean of 33.88 ± 12.43%, while the level of sperm with damaged chromatin (Fig 1) was 4.17 ± 2.96%. Spearman's correlation test revealed a positive correlation between percentage of live sperm and motility (r=0.77217, p=0.0002), whereas the percentage of damaged chromatin had a negative correlation with viability (r=-0.43104, p=0.0087) and motility (r=-0.48337, p=0.0421).
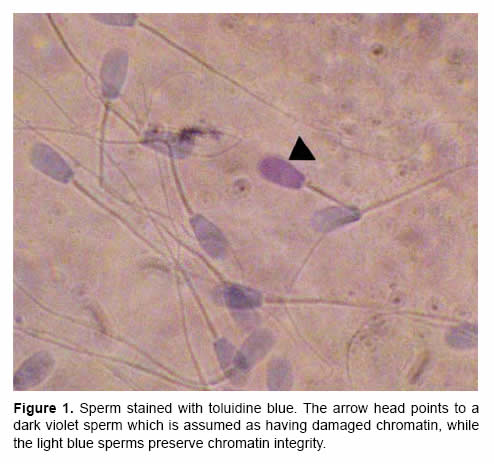
Discussion
In the present study, the percentage of cryopreserved sperm with damaged chromatin using TB stain and its relationship with viability and motility were determined. Little information is available about this subject in bulls under tropical conditions. Recently, Zúccari et al. (2008), using TB stain, showed that cryopreserved Nellore semen had 3.15 ± 1.74% of sperm with damaged chromatin, a result lower than the observed in the present study. Some studies suggest that the presence of sperm with damaged chromatin in cryopreserved semen could be a consequence of the cryopreservation process (Khalifa et al., 2008; Slowínska et al., 2008), but the effect of cryopreservation on sperm DNA integrity, even though significant, seems to be low when measured through the comet assay (Slowínska et al., 2008).
The level of damaged chromatin was affected significantly by bull effect, and this is in agreement with Gillan et al. (2008) and Khalifa et al. (2008). Evaluation of sperm chromatin integrity alone (Madrid-Bury et al., 2005) or in combination with other parameters (Gillan et al., 2008) could explain fertility variations between bulls to select superior bulls. More sperm with damaged chromatin were observed in poorly fertile bulls (Beletti and Mello, 1996; Vieytes et al., 2008). In previous studies, a negative correlation between sperm chromatin integrity and other parameters of sperm quality was observed (Januskauskas et al., 2003; Kasimanickam et al., 2006; Khalifa et al., 2008). The correlation coefficient observed in the present study suggests that sperm chromatin integrity, viability and motility could be associated but are not concomitant, indicating that live or motile sperm could or not carry damaged chromatin. Thus sperm chromatin damage could be used independently to evaluate sperm quality. Additionally, the results could explain the negative association between sperm chromatin damage and fertility observed in several studies (Ballachey et al., 1987; Dobrinski et al., 1994; Madrid-Bury et al., 2005; Fatehi et al., 2006; García-Macías et al., 2008).
In this study, TB was used to identify sperm with damaged chromatin. Dye variations of stained sperm on the slide diminish the repeatability when compared with other methods for evaluating sperm chromatin. Therefore, studies comparing TB stain with other methods for evaluating sperm chromatin integrity are necessary. In humans, TB showed high correlation coefficients with SCSA, TUNEL and AO (Erenpreisa et al., 2003; Erenpreiss et al., 2004). However, in the case of bull semen, there is a lack of studies comparing these techniques. In cryopreserved semen of buffaloes (Bubalus bubalis), Melo and Beletti (2002) observed a moderate correlation between TB and acridine orange (r=0.38). Additionally, TB was considered more sensitive and less subjective in identifying sperm with abnormal chromatin than acridine orange, although both methods had similar stability and reliability. Additionaly, the TB stain is a practical methodology to study sperm morphometric features and to identify nuclear regions susceptible to chromatin alterations (Beletti et al., 2004; Beletti et al., 2005).
In conclusion, cryopreserved semen from Brahman bulls has low level of sperm with damaged chromatin, measured with the TB stain. Damaged chromatin is negatively correlated with viability and motility and could be used to estimate sperm quality. Further studies to compare toluidine blue stain with other methods are needed.
Acknowledgement
This work was sponsored by Council of Scientific and Humanistic Development of Zulia University (CONDES-LUZ, grant CC-0307-09) and Fundo La Rosita (El Guayabo, Zulia-Venezuela).
References
1. Agarwal A, Said TM. Sperm chromatin assessment. In: Gardner DK, Weissman A, Howles CM, and Shoham Z, editors. Textbook of ART, 2nd ed. Taylor & Francis Group, London: UK; 2004 p. 93-106.
2. Ballachey BE, Hohenboken WD, Evenson DP. Heterogeneity of sperm nuclear chromatin structure and its relationship to bull fertility. Biol Reprod 1987; 36:915-925.
[ Links ]3. Beletti ME, Mello ML. Methodological variants contributing to detection of abnormal DNA-protein complexes in bull spermatozoa. Braz J Gen 1996; 19:97-103.
[ Links ]4. Beletti ME, Mello ML. Comparison between the toluidine blue stain and the feulgen reaction for evaluation of rabbit sperm chromatin condensation and their relationship with sperm morphology. Theriogenology 2004; 62:398-402.
[ Links ]5. Beletti ME, Costa L, Mendes M. Morphometric features and chromatin condensation abnormalities evaluated by toluidine blue staining in bull spermatozoa. Braz J Mophol Sci 2005; 22:85-90.
[ Links ]6. Bochenek M, Smorag Z, Pilch J. Sperm chromatin structure assay of bulls qualified for artificial insemination. Theriogenology 2001; 56:557-567.
[ Links ]7. Celeghini ECC, De Arruda RP, De Andrade AFC, Nascimento J, Rafhael CF, Mazza PH. Effects that bovine sperm cryopreservation using two different extenders has on sperm membranes and chromatin. Anim Reprod Sci 2008; 104:119-131.
[ Links ]8. Charles-Ostermeier G, Sargeant GA, Yandell BS, Evenson DP, Parrish JJ. Relationship of bull fertility to sperm nuclear shape. J Androl 2001; 22:595-603.
[ Links ]9. Dobrinski I, Hughes HP, Barth AD. Flow cytometer and microscopic evaluation and effect on fertility of abnormal chromatin condensation in bovine sperm nuclei. J Reprod Fertil 1994; 101:531-538.
[ Links ]10. Eid LN, Lorton SP, Parrish JJ. Paternal influence on S-phase in the first cell cycle of the bovine embryo. Biol Reprod 1994; 51:1232-1237.
[ Links ]11. Erenpreisa J, Erenpreiss J, Freivalds T, Slaidina M, Krampe R, Butikova J, Ivanov A, Pjnova D. Toluidine blue test for sperm DNA integrity and elaboration of image cytometry algorithm. Cytometry Part A 2003; 52A:19-27.
[ Links ]12. Erenpreiss J, Jepson K, Giwercman A, Tsarev I, Erenpreisa J, Spano M. Toluidine blue cytometry test for sperm DNA conformation: comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum Reprod 2004; 19:2277-2282.
[ Links ]13. Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male fertility and comparisons with other techniques. J Androl 2002; 23:25-43.
[ Links ]14. Fatehi AN, Schoevers E, Roelen BAJ, Colenbrander B, Gadella BM. DNA damage in bovine sperm does not block fertilization and early embryonic development but induces apoptosis after the first cleavages. J Androl 2006; 27:176-188.
[ Links ]15. García-Macías V, De Paz P, Martinez-Pastor F, Álvarez M, Gomes-Alves S, Bernardo J., Anel E, Anel L. DNA fragmentation assessment by flow cytometry and Sperm- Bos-Halomax (bright-field microscopy and fluorescence microscopy) in bull sperm. Int J Androl 2007; 30:88-98.
[ Links ]16. Gillan L, Kroetsch T, Chis Maxwell WM, Evans G. Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim Reprod Sci 2008; 103:201-214.
[ Links ]17. Hallap T, Nagy S, Haard M, Jaakma U, Johannisson A, Rodríguez-Martínez H. Sperm chromatin stability in frozen-thawed semen in maintained over age in AI bulls. Theriogenology 2005; 63:1752-1763.
[ Links ]18. Hansen PJ. Embryonic mortality in cattle from the embryo's perspective. J Anim Sci 2002; 80:E33-E44.
[ Links ]19. Januskauskas A, Johannisson A, Rodríguez-Martínez H. Assessment of sperm quality through fluorometry and sperm chromatin structure assay in relation to field fertility of frozen-thawed semen from Swedish AI bulls. Theriogenology 2001; 55:947-961.
[ Links ]20. Januskauskas A, Johannisson A, Rodríguez-Martínez H. Subtle membrane changes in cryopreserved bull semen in relation with sperm viability, chromatin structure, and field fertility. Theriogenology 2003; 60:743-758.
[ Links ]21. Kasimanickam R, Nebel RL, Peeler ID, Silvia WL, Wolf KT, McAllister KT, Cassell BG. Breed differences in competitive indices of Holstein and Jersey bulls and their association with sperm DNA fragmentation index and plasma membrane integrity. Theriogenology 2006; 66:1307-1315.
[ Links ]22. Khalifa TA, Rekkas CA, Lymberopoulos AG, Sioga A, Dimitriadis I, Papanikolaou T. Factors affecting chromatin stability of bovine spermatozoa. Anim Reprod Sci 2008, 104:143-163.
[ Links ]23. Kosower NS, Katayose H, Yanagimachi R. Thiol-disulfide status and acridine orange fluorescence of mammalian sperm nuclei. J Androl 1992; 13:342-348.
[ Links ]24. Madrid-Bury N, Pérez-Gutiérrez JF, Pérez-Garnelo S, Moreira P, Pintado-Sanjuanbenito B, Gutiérrez-Adan A, De La Fuente Martínez J. Relationship between non-return rate and chromatin condensation of deep frozen bull spermatozoa. Theriogenology 2005; 64:232-241.
[ Links ]25. Mello M, Beletti ME. Methods for abnormal spermatozoa chromatin condensation identification in buffaloes (Bubalus bubalis). Bubalus bubalis 2002; 1:57-65.
[ Links ]26. Mello MS. Induced metachromasia in bull spermatozoa. Histochemistry 1982; 74:387-392.
[ Links ]27. Mukhopadhyay CS, Gupta AK, Yadav BR, Chauhan IS, Aparna Gupta, Mohanty TK, Raina VS. Effect of cryopreservation on sperm chromatin integrity and fertilizing potential in bovine semen. Livestock Sci 2010; In press.
[ Links ]28. Oliva R. Protamines and male fertility. Hum. Reprod Update 2006; 12:417-435.
[ Links ]29. Sardoy MC, Carretero MI, Neild DM. Evaluation of stallion sperm DNA alterations during cryopreservation using toluidine blue. Anim Reprod Sci 2008; 107:349-350.
[ Links ]30. Slowínska M, Karol H, Ciereszko A. Comet assay of fresh and cryopreserved bull spermatozoa. Cryobiology 2008; 56:100-102.
[ Links ]31. Tamuli M, Watson P. Use of a simple staining technique to distinguish acrosomal changes in the live sperm sub-population Anim Reprod Sci 1994; 35:247-254.
[ Links ]32. Thomson LK, Fleming SD, Aitken RJ, De luliis GN, Zieschang JA, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod 2009; 24:2061-2070.
[ Links ]33. Urrego R, Ríos A, Olivera Ángel M, Camargo O. Efecto de la centrifugación sobre la membrana plasmática y el ADN de espermatozoides bovinos. Rev Colomb Cienc Pecu 2008; 21:19-26.
[ Links ]34. Waterhouse KE, Haugan T, Kommisrud E, Tverdal A, Flatberg G, Farstad W, Evenson DP, De Angelis PM. Sperm DNA damage is related to field fertility of semen from young Norwegian red bulls. Reprod Fertil Dev 2006; 18:781-788.
[ Links ]35. Zini A, Bielcki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation in fertile and infertile men. Fertility and Sterility 2001; 75:674-671.
[ Links ]36. Zúccari CESN, Carrijo PR, Leite PA, Scaldeal PR, Rodovalho NC, Zanenga CA, Kiefer C, Silva EV. Seleção em gradiente de Percoll sobre os parâmetros espermáticos do sêmen bovino congelado. Rev Bras Saúde Prod An 2008; 9:358-366.
[ Links ]













