Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.38 no.4 Bogotá Oct./Dec. 2010
ArtÃculo de Revisión
Early Coagulopathy in Trauma: Do Coagulopathic Patients Reach the Operating Room?
CoagulopatÃa temprana en trauma:
¿Llegan los pacientes coagulopáticos
a la sala de cirugÃa?
Juan Carlos Jiménez B.*, Jairo de La Peña L.**, Rubén Teherán M.***, Andrés Orozco****
* MD Especialista en AnestesiologÃa, Universidad de Cartagena. Anestesiólogo Hospital
Universitario del Caribe, Cartagena, Colombia. juanjim170@hotmail.com
** Residente de I año CirugÃa General, Universidad de Cartagena, Cartagena, Colombia.
*** MD. Anestesiólogo director UCI Hospital Universitario del Caribe, Cartagena, Colombia.
**** Médico de Planta, UCI Hospital Universitario del Caribe, Cartagena, Colombia.
Recibido: enero 25 de 2010. Enviado para modificaciones: enero 28 de 2010. Aceptado: julio 26 de 2010.
SUMMARY
Introduction. Acute coagulopathy in trauma results in multiple complications such as the need for blood products, higher rates of organ dysfunction, longer stay in the ICU and higher mortality. With the current knowledge of the pathophysiology of trauma and of the cellular coagulation pathway it is now possible to improve diagnosis and treatment of the initial coagulopathy and achieve better outcomes in our trauma centers.
Methods. This paper looks into the basic physiology of coagulation, and the etiology, diagnosis and treatment of early coagulopathy in trauma. The search was done using Mesh and non-Mesh terms with AND connectors: Anesthesia-coagulopathy, postinjury and trauma thromboelastography, transfusion and trauma, shock-Mechanism and trauma review.
Results. Acute or early coagulopathy in trauma is directly associated with a state of shock and is characterized by anticoagulation and systemic hyperfibrinolysis; protein C is known to be implicated in this process. It has also been determined that six multi-factorial pathophysiological mechanisms may perpetuate coagulopathy in trauma patients, namely, inflammation, acidosis, hypothermia, shock, tissue trauma and hemodilution. Diagnosis is made using the different tests (PT, PPT, platelets) that have been in use for a long time; however, these tests have drawbacks that limit their clinical usefulness. Thromboelastography can now help guide early transfusion using the best proportion of red blood cells, plasma and platelets on the basis of the best available evidence.
Conclusions. We have some knowledge about the pathophysiology coagulopathy associated with trauma but more research in this field is needed. Rapid diagnosis and immediate intervention are important to improve the outcomes with our patients.
Keywords: Coagulopathy, trauma, hemorrhage, transfusion, thromboelastography (Source: MeSH, BIREME)
RESUMEN
Introducción. De la coagulopatÃa aguda en el trauma, resultan múltiples complicaciones como la necesidad de administración de hemoderivados, mayor incidencia de disfunción orgánica, aumento de estancia en unidad de cuidados intensivos y mayor mortalidad. Con el conocimiento actual de la fisiopatologÃa del trauma y la vÃa celular de la coagulación es ahora posible mejorar el dignóstico y tratamiento de la coagulopatÃa inicial y conseguir mejores resultados en nuestros centros.
Métodos. Este artÃculo examina la fisiologÃa básica de la coagulación, la etiologÃa, el diagnóstico y el tratamiento de la coagulopatÃa temprana en trauma. La búsqueda se realizó con términos Mesh y no Mesh con conectores AND: Anesthesia-coagulophaty, postinjury and trauma thromboelastography, transfusion and trauma, shock-Mechanism and trauma review.
Resultados. La coagulopatÃa aguda o temprana en trauma está directamente asociada al estado de shock y se caracteriza por anticoagulación e hiperfibrinolisis sistémica; hay evidencia de la implicación de la proteÃna C en este proceso.
Se ha establecido que seis mecanismos fisiopatológicos multifactoriales pueden perpetuar la coagulopatÃa en los pacientes traumatizados; éstos son: inflamación, acidosis, hipotermia, shock, trauma tisular y hemodilución. El diagnóstico se realiza con las diferentes pruebas (TP, TPT, plaquetas) ya conocidas desde hace mucho tiempo, pero con limitaciones que reducen su utilidad clÃnica. Ahora la tromboelastografÃa nos puede ayudar a guiar la transfusión, con el concepto actual de transfusión temprana de glóbulos rojos, plasma y plaquetas, utilizando la mejor proporción según la evidencia disponible.
Conclusiones. Contamos, con algún conocimiento sobre la fisiopatologÃa de la coagulopatÃa asociada con trauma pero son necesarias más investigaciones, en este campo. El diagnóstico rápido y una intervención directa inmediata son importantes para mejorar el desenlace de nuestros pacientes.
Palabras claves: CoagulopatÃa, trauma, hemorragia, transfusión, tromboelastografÃa (Fuente: DeCS, BIREME)
INT RODUCTION
Mortality in patients with trauma of any etiology is directly related to disorders of the coagulation pathway. Coagulation is an integral part of inflammatory conditions and, when activated, it may result in greater susceptibility to the onset of sepsis (1-3). It has been recently established that coagulopathy occurs in one out of every four trauma patients admitted to trauma centers, and this translates into a four-fold increase in mortality. (4,5) Mortality is high in cases of trauma associated with massive bleeding, and more than 50 % of patients with massive bleeding go on to die. (6) Hemorrhage is the primary cause of death in trauma patients, and 40% develop coagulopathy with a fatal outcome. In a study conducted in a trauma center in Miami, Florida (7) revealed a 28 % rate of coagulation profile abnormalities in admitted trauma patients, and early coagulopathy was associated with poor survival rates. Brohi et al. (8), showed that early abnormal PT and PPT in trauma patients is an independent factor associated with mortality. Traditionally, the thinking has been that early coagulopathy in trauma patients results from the use of clotting factors, the dilution caused by intravenous fluids, and the presence of hypothermia and metabolic acidosis. However, it has been demonstrated recently that none of these factors is responsible for the early coagulopathy that occurs initially after trauma or at a later time. It is current knowledge that tissue hypotension and hypoperfusion injury may activate the thrombomodulin- protein C pathway, triggering the process of coagulopathy and fibrinolysis (9-11). Current evidence suggests that the prompt adequate management of coagulopathy in trauma results in lower mortality and reduced severity of late complications (12). The purpose of this review is to underscore the importance of these new concepts of early coagulopathy in trauma, the coagulation cascade, diagnosis and initial treatment, and their application in our operating rooms and emergency services.
BASIC COAGULATION MECHANISM
The classical coagulation model, introduced in 1964 as the âcoagulation cascadeâ (Figure 1), has been replaced with a cellular model that emphasizes the interaction of coagulation proteins with the cell surface of subendothelial platelets and the endothelium (12-14).
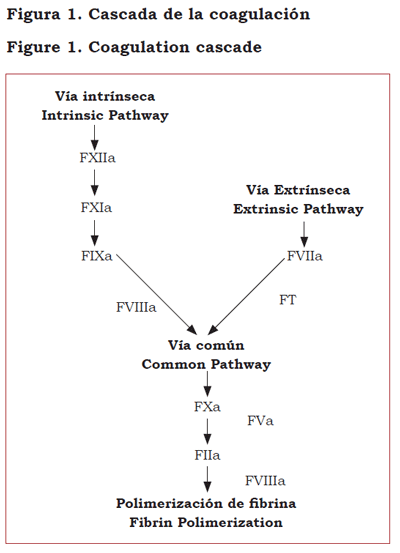
According to this new model, hemostasis starts with the formation of a complex between the tissue factor (TF) (expressed in subendothelial fibroblasts as a result of the damage to the vascular wall) and the activated factor VII normally present in low concentrations in blood circulation. The TF-factor VIIa complex converts factor X into activated factor X which, in turn, activates prothrombin into thrombin. There are two inhibitors involved in this initial phase:
1. The tissue factor pathway inhibitor (TFPI) that neutralizes the FXa when it is in the TF-FVIIa complex form.
2. Antithrombin III that circulates in high concentrations (150 μg/ml) and neutralizes FXa and thrombin.
Thrombin activates platelets and factors VIII, V and XI. Platelets change shape as a result of the long exposure to the negative charge of phospholipids that serve as a matrix for fibrin formation from fibrinogen through the action of thrombin. Factors VIIIa and Va act as cofactors for FIXa and Fxa, respectively. This process may be divided into three steps: initiation, propagation and amplification (Figure 2). An intact endothelium has multiple anticoagulation functions, including the release of nitric oxide, prostacycline and ADPase, all of which down-regulate platelet activity. TFPI is released by the endothelium very soon after heparin administration, thus diminishing the action of the TF-FVIIa complex. Thrombomodulin is another anticoagulant and anti-inflammatory protein that binds to the endothelial cell.
Tissue plasminogen activator (TPA) is released in response to a pro-coagulant stimulus in order to promote fibrinolysis. Coagulation factors are consumed at a basal rate in the absence of trauma, just like there is basal thrombin production (14). The hemostasis response is confined to the injury site as a result of local activation of adhered platelets, and activated factors are quickly neutralized as follows: TF-FVIIa-FXa by TFPI and Fxa, and thrombin by antithrombin. Thrombomodulin and thrombin bind to the endothelial cell, activating protein C (PC), the natural anticoagulant that inhibits cofactors VIIIa and Va and stimulates TPA release by the endothelial cell. Protein S contributes to PC action. Moreover, PC has been said to have cryoprotective and anti-inflammatory effects. The continuous release of FXa, fibrin and thrombin leads to the activation of the fibrinolytic system (Figure 2).
ACUTE COAGULOPATHY MECHANISM IN TRAUMA
Coagulopathy in trauma has been classically described as the result of dilution, hypothermia and acidemia associated with the consumption or dysfunction of clotting factors. Causes are multifactorial, but there are certain predominant mechanisms associated with the clinical condition of the patient, including tissue injury, shock, hemodilution, hypothermia, acidemia and inflammation (15); the latter being responsible, together with the activation of coagulation in the increased susceptibility to subsequent sepsis.
Injury severity is associated with the degree of coagulopathy (16). Based on new theories about the physiology of coagulation, we may consider that type III collagen exposure leads to platelet aggregation and factor VII activation as triggers of the coagulation process (17). It has been determined that initial shock may be the trigger of the coagulopathy, and it is an independent mortality factor in trauma (18). Hemodilution plays a critical role in coagulopathy associated with trauma (19,20). The effect of fluids on coagulation has been widely demonstrated in mathematical and animal models (21,22). The type of resuscitation fluids may influence coagulation disorders in trauma; different colloids are known to interfere with clot formation and stability. The function of platelets and coagulation factors is inhibited by hypothermia (23,24); factor VII is known to retain only 50 % of its activity at 28 °C (25,26). Platelets are the most vulnerable to drops in body temperature, as they lose their adhesiveness at temperatures below 30 °C (27). Mortality increases in trauma patients when the body temperature drops below 32 °C (28,29). Acidemia observed in trauma patients may be due to the state of shock and the use of sodium chloride during resuscitation; the activity of clotting factors, FXa/Va in particular, is markedly diminished because of this acidemia which in turn increases fibrinogen degradation (30).
A pH under 7.2 has deleterious effects on proteases but it may be corrected using buffer solutions, although the same is not true of the coagulopathy (1).
Finally, it is important to remember the relationship between coagulation factors and the induction of inflammation and of the immune response through the activation of endothelial cells. Inflammation leads to a coagulation abnormality (31,32) consisting of increased initial bleeding followed later by hypercoagulability.
All of these factors are implicated in trauma-associated coagulopathy as acquired systemic coagulopathy, but we will now analyze how this process starts within the first few minutes: Brohi et al. (11) conducted a prospective study in one trauma center taking blood samples of 208 patients within the first 10 minutes of their arrival at the emergency room in order to measure PT, PPT, D dimer, fibrinogen, thrombomodulin, protein C, plasminogen tissue activator inhibitor and blood gases. The aim of the study was to determine the underlying base excess or deficit. PT and PPT prolongation was observed only in patients with high BE levels. These patients were shown to have high levels of thrombomodulin, and D dimer, and low levels of protein C and plasminogen tissue activator inhibitor, suggesting a hyperfibrinolytic state. Moreover, high thrombomodulin levels and low protein C levels were associated with high mortality and transfusion rates, and more days in mechanical ventilation. In their work on coagulopathy and head injury with 39 patients, Cohen et al. concluded that, in patients with head injury, coagulopathy does not result only from the release of tissue factor by the brain but also from severe hypoperfusion and protein C activation that acts as the triggering factor. In a later study, Brohi et al. (10). confirmed that acute coagulopathy in trauma may be directly associated with tissue hypoperfusion, and is characterized by anticoagulation and hyperfibrinolysis. Protein C is activated together with thrombomodulin and the protein C endothelial receptor. (Figure 3.) Once activated, protein C exerts an anticoagulant effect and inhibits co-factors VIIIa and Va irreversibly. The protein S co-factor is required for this reaction; protein C has a direct inhibitory effect on fibrin formation by blocking the plasminogen tissue activator inhibitor -1, leading to plasminogen conversion into plasmin and giving rise to a hyperfibrinolytic state that can now be detected using thromboelastrography (33). More recently, ChesBEro et al. (32) in a rat model of induced trauma and hemorrhage, published results in favor of the protein Cmechanism and the initial state of shock as triggers of early coagulopathy in trauma.
DIAGNOSIS OF COAGULOPATHY IN TRAUMA
In most initial emergency care centers the only laboratory tests requested include prothrombin time (PT), partial activated prothrombin time (PPT), with several time limitations (20-60 minutes to get the result) and difficulties in assessing platelet function. Those tests are based on the old coagulation cascade model (intrinsic and extrinsic pathways); therefore, they do not offer any information about the interaction between platelets and coagulation factors (14) or about the stability of the thrombus, because these tests are concluded before the clot is stabilized by the action of FXIIIa.
In contrast with PT and PPT, thromboelastography permits the functional assessment of the fibrinolytic and factor XIII system. In trauma patients, PPT appears to be more specific than PT in predicting outcome. (1) In the study by Brohi (11) there was a higher correlation between PPT and lower levels of protein C due to the effects of protein C on co-factors V and VIII. Thromboleastography was assessed in a study with 90 trauma patients and it may be used for quick detection of coagulation alterations (33). The delay in obtaining the results in coagulation tests, may be the originator, to implement emergency transfusion protocol (34).
Tests currently available in our services miss pathophysiological aspects of coagulation in trauma patients. Bearing in mind the potential cause of the onset of acute coagulopathy in trauma, it is necessary to assess arterial and venous gases. Moreover, it is necessary to make serial measurements of mixed venous oxygen saturation and to determine the BE and serum lactate values as measurements of hypoperfusion (35,36). BE during resuscitation correlates well with transfusion requirements, length of stay in the ICU and serum lactate levels.
TREATMENT OF COAGULOP ATHY IN TRAUMA
In trauma, most patients who come to the emergency rooms present multiple associated issues (36). Consequently, during resuscitation, management must focus on the cause of the injury, and the main issue to address is bleeding as a source of shock (37). The best outcomes are obtained with an objectives-based approach with constant assessment of each intervention. This is where the early objectives determined by Dr. Rivers in his study of patients in septic shock may be applied. These are objectives that can be extrapolated for patients in hypovolemic hemorrhagic shock, without neglecting the critical need to control bleeding as the most important pillar in resuscitation. On the basis of the hemodynamic profile at the time of admission, delayed resuscitation must be considered until vascular control of the bleeding site is achieved, with early identification of signs and symptoms of hypoperfusion or shock.
According to the American College of Surgeons (38) blood derivatives may be used as first-line management to regain hemostatic control, including packed red blood cells, platelets and fresh frozen plasma. Fibrinogen levels under 100 mg/dl are an indication for the use of up to 10 U of cyroprecipitate. At this point the thromboelastography may help guide transfusion (39).
The exclusive use of packed red blood cells may be associated with hemodilution due to the lack of clotting factors as a proportion of total blood volume. Hence the imperative need to use fresh plasma and platelets in a 1:1 proportion.
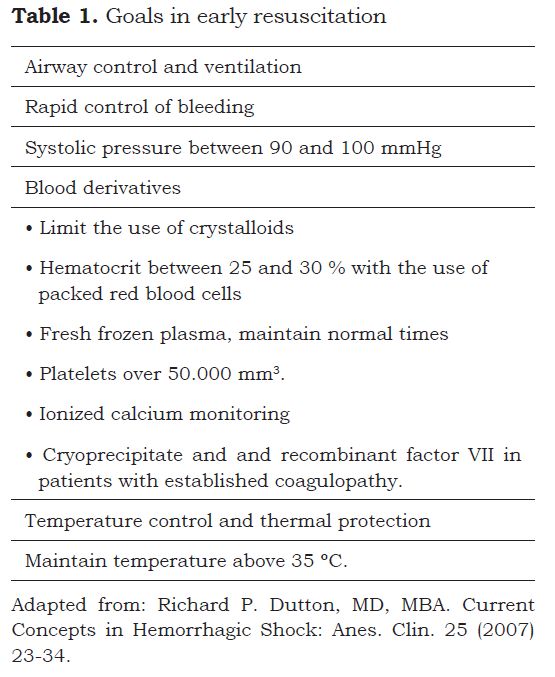
Coagulopathy may be reversible once hemostatic control is achieved and the hemodynamic state is recovered. Early goals have to be established in resuscitation (37) (table 1). It is important to stress that there are multiple causes of bleeding in trauma and critically-ill patients: (40,41):
1. Dilutional coagulopathy due to fluid replacement and dilution of clotting factors.
2. Multiple red blood cell transfusions with no platelet or clotting factor replacement.
3. Trauma per se.
4. Pre-existing medical conditions.
5. Acquired coagulopathies (acidosis, hypothermia and hypocalcemia).
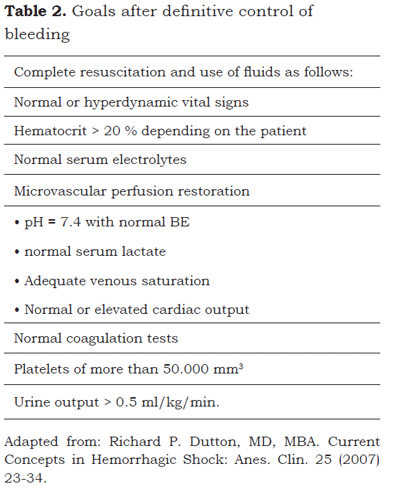
Once hemostatic and vascular control are achieved, thermal and perfusion maintenance goals must be established and acidosis addressed. Other goals can be established after vascular control is achieved and a more aggressive resuscitation should be instituted in order to control and prevent the other two factors of the death triad, namely, acidosis and hypothermia (table 2).
As confirmed by the review of the pathophysiology of hemostasis and its various components, inadequate resuscitation, poor control of bleeding, persistent hypoperfusion and inadequate protection against hypothermia during early management open the door to the deadly triad as trigger of a fatal outcome in these patients. The experience described by many authors has led to realize that, regrettably, once coagulopathy is established in a trauma patient, despite early replacement of blood derivatives and hypoperfusion compensation, there is big gap in the management of these problems, and a need to address the coagulopathic state through the stimulation of endogenous procoagulant substances.
A new medication is now available that many have claimed to produce miraculous results in the control of coagulopathy, namely, activated recombinant factor VII (rFVIIa) or NovoSeven (Novo Nordisk, Bagsvaerd, Denmark).
Since it was first used in the 1980âs to treat hemophiliacs it has been approved in Europe and by the United States Food and Drug Administration (FDA) for this indication.
At present there is a large number of publications regarding the use of this type of substance in the management of coagulopathy. In isolated cases it has been used successfully to reverse coagulopathy in patients treated with warfarin. (41) Consequently, it is important to underscore the role of the rFVIIa in the coagulation cascade: after the tissue factor-dependent factor VII is activated in the subendothelium, it leads to the production of high levels of thrombin. The action of the rFVIIa is of the utmost importance because it promotes coagulation at the site of injury and not at a systemic level; likewise, it has also been shown to increase the density of the clot, thus increasing its resistance to fibrinolysis, and reducing the number of units of red blood cells for transfusion (42). There is no consensus regarding the adequate dose of the rFVIIa, but the recommended range is 40-90 μg/kg. (43) Moreover, there are no management guidelines to help define the precise indications and contraindications in these types of patients, and the use of thromboelastrography may be of help (44).
Concentrated prothrombin complexes (CPC) are highly purified, have a hemostatic activity and are prepared from plasma. They contain so-called vitamin K-dependent factors (II, VII, IX and X) that are indicated for use in reverting anticoagulation from oral anticoagulants, and are used sometimes to manage coagulopathies caused by FII or FX deficiencies (45). In some European countries they are used to manage massive peri-operative and post-operative bleeding (46). CPCs must have a minimum level of FIX, FII and FX potency, closer to that of FIX, and a lower FVII potency (47); and the concentration of clotting factors is much higher than the plasma concentration. The main indication for their use is vitamin K-dependent factor deficiencies or congenital factor II and X deficiencies, although they should be used only in life-threatening bleeding and together with vitamin K (48).
The only absolute contraindication for their use is in disseminated intravascular coagulation (DIC), and they must be used with care in patients with type II heparin-induced thrombocitopenia or with heparin-mediated thrombotic complications.
CONCLUSIONS
The approach to patients with severe trauma must focus on a prompt and adequate initial management in the emergency room, with appropriate resuscitation, protection from hypothermia and maintenance of perfusion, on the basis of the new pathophysiological concepts. Vascular control to prevent exanguination as a contributor to the deadly triad must go hand in hand with the initial management and requires the participation of an anesthesiologist and a surgeon. The decision to give blood products early on is important, as is also the maintenance of pre and post-vascular control goals in resuscitation. Moreover, it is important to assess the different interventions quantitatively until all the goals are met. We must not forget that trauma induces an inflammatory response, and this can also be initiated by coagulation proteases (49), leading into the patient to a prothrombotic state, similar to that observed in severe sepsis (50).
Diagnostic tests available in our emergency departments have their limitations. Thromboelastography is emerging as a diagnostic tool and as a guide for therapy, but additional randomized clinical trials are required to assess its use.
Although the adequate and timely use of rFVIIa in the treatment of trauma is promising, there are still no protocol parameters relative to precise indications or contraindications.
It is worth noting that the use of rFVIIa has improved the control of intractable bleeding due to coagulopathy in seriously injured patients, and it is important to consider this option for the control of coagulopathy in order to address acidosis
and hypothermia as perpetuating factors.
References
1. Brohi K, Mitchell J, Cohenb, Ross A, Davenporta. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680â685.
2. Charles A, Shaikh AA, Walters M, Huehl S, Pomerantz R. Bloodtransfusion is an independent predictor of mortality after blunttrauma. Am Surg. 2007;73:1â5.
3. Kheirabadi BS, Crissey JM, Deguzman R, Holcomb JB. In vivobleeding time and in vitro thrombelastography measurements are better indicators of dilutional hypothermic coagulopathy thanprothrombin time.J rauma.2007;62:1352â1359
4. Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography.JThrombHaemost. 2007;5:289 â295.
5. Borgman MA, Spinella PC, Perkins JG, et al. The ratio of bloodproducts transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805â 813.
6. Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a Reassessment. J Trauma. 1995; 38(2):185-193.
7. MacLeod JB, Lynn M, McKenney M, Cohn S, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003; 55(1):39-44.
8. Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J.Trauma.2003;54(6):1127-1130.
9. Stahel PF,Moore EE,Schreier SL, Flierl MA,Kashuk JL. Transfusion Strategies in Posinjury Coagulophaty. Curr Opin Anesth. 2009;22:289-298.
10. Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma.2008; 64:1211â1217.
11. Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg.2007; 245:812â81.
12. Torres JC, Torres MU, Benjumea MA. Manejo de hemorragia digestiva profusa secundaria a hemangioma intestinal con factorVIIa recombinante [Reporte de caso y revisión de la literatura]. Act Colom de Cuid Inten. 2008; 8(4): 322-329.
13. Tanaka, kenichi A, Keil NS, Levy, Jerrold H. Blood Coagulation: Hemostasis and Thrombin Regulation. Anesth. & Analg. 2009 May 108(5) :1433-1446.
14. John RH, Brohi K, Richard PD,Carl JH, John BH , Kluger Y,Mackway K, Par M, , Rizoli SB , Yukioka T , Hoyt DB, Bouillon B. The Coagulopathy of Trauma: A Review of Mechanisms. J Trauma. 2008;65:748 â754.
15. Esmon CT. The interactions between inflammation and coagulation.Bj J Haematol.2005;131:417-430.
16. Mann KG. Biochemistry and physiology of blood coagulation.Thromb Haemost. 1999;82:165â174.
17. Siegel JH, Rivkind AI, Dalal S, et al. Early physiologic predictors of injur Severity and death in blunt multiple trauma. Arch Surg 1990; 125:498â508.
18. Brummel ZK, Whelihan MF, Ziedins EG, Mann KG. Theresuscitative fluid you choose may potentiate bleeding. J Trauma. 2006;61:1350 â1358.
19. Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation?. Am J Surg 2005;190:479â484.
20. Hirshberg A, Dugas M, Banez EI, Scott BG, Wall MJ, Mattox KL. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454â463.
21. Brazil EV, Coats TJ. Sonoclot coagulation analysis of in-vitro haemodilution with resuscitation solutions. J R Soc Med 2000;93:507â510.
22. Vincent JL, Rossaint R, Riou B, Ozier Y, Zideman D, Spahn DR. Recommendations on the use of rFVIIa as an adjunctive treatment for massive bleeding -a European perspective. Critical Care 2006; 10: R120.
23. Reed RL II, Bracey AW, Jr., Hudson JD, Miller TA, Fischer RP.Hypothermia and blood coagulation: dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990;32:141â152.
24. Wolberg AS, Meng ZH, Monroe DM III, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56:1221â1228.
25. Meng ZH, Wolberg AS, Monroe DM III, Hoffman M. The effect oftemperature and pH on the activity of factor VIIa: implications forthe efficacy of high-dose factor VIIa in hypothermic and acidoticpatients. J Trauma. 2003;55:886â891.
26. Wolberg AS, Meng ZH, Monroe DM, Hoffman MA. systematic evaluation ofthe effect of temperature on coagulation enzyme activity and platelet function.J Trauma 2004; 56:1221â1228.
27. Martini WZ, Pusateri AE, Uscilowicz JM, DelgadoAV, HolcombJB. Independent contributions of hypothermia and acidosis tocoagulopathy in swine. J Trauma. [discussion] 2005;58:1009â1010.
28. Cosgriff N, Moore EE, Sauaia A,Moynihan K, Burch JM,Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. [discussion] 1997;42:857â 861.
29. Martini WZ, Dubick MA, Wade CE,Holcomb JB. Evaluation of tris-hydroxymethyl aminomethane on reversing coagulation abnormalities caused by acidosis in pigs. Crit Care Med. 2007;35:1568 â1574.
30. Levi M, van der Poll T, ten Cate H, van Deventer SJ. The cytokine mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. 1997;27:3â9.
31. Shebuski RJ, Kilgore KS. Role of inflammatory mediators in thrombogenesis. J Pharmacol Exp Ther. 2002;300:729 â735.
32. Chesebro BB, Rahn P, Carles M,Esmon C, Jun X, Brohi K, Frith D, Pittet J-P, Cohen M. Increase in activated protein c mediates acute traumatic coagulopathy in mice. Shock. 2009 Dec 32;6:659-665.
33. Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalitiesin trauma patients by rotation thrombelastography. J Thromb Haemost 2007; 5:289â295.
34. Devenport RA, Brohi K. Coagulophaty in trauma patients : Importance of thrombocyte function?.Curr Opin Anesth.2009;22:261-266.
35. Oliver M. Theusinger D R, Ganter M. Transfusion in trauma: why and how should we change our current practice? Curr Opn Anaesth 2009,22:000â000
36. Feliciano DV, Mattox K, Moore E. Trauma, 6th Edition: McGraw-Hill ;2008.
37. Dutton RP. Current Concepts in Hemorrhagic Shock. Anesth Clinics 2007 March 1; 25 :23-34.
38. American College of Surgeons Committee on Trauma: Advanced Trauma Life Support(ATLS)Course for Physicians,edn 7.Chicago:American College of Surgeons;2008.
39. Sorensen B, Ingerslev J. Tailoring haemostatic treatment to patient requirements - an update on monitoring haemostatic response using thrombelastography. [Suppl] Haemophilia 2005 11; 1:1-6.
40. Brown RB, Klar J, Teres D, Lemeshow S, Sands M. Prospective study of clinical bleeding in intensive care unit patients. Crit Care Med. 1988 Dec 16(12):1171-6.
41. Stein D, Dutton R, Kramer M, Scalea T. Reversal of Coagulopathy in Critically Ill Patients With Traumatic Brain Injury: recombinant Factor VIIa is More Cost-Effective Than Plasma. J Trauma 2009;66: 63â75.
42. Galvan DA,Fink MP. Recombinant factor VIIa in severe trauma: Further study needed. Crit care 2006 10:308.
43. Martinowitz U, Holcomb JB, Pusateri AE, et al. Intravenous rFVIIa administered for hemorrhage control in hypothermic coagulopathic swine ith grade V liver injuries. J Trauma 2001; 50:721â729.
44. Bartal C, Yitzha A. The role of thromboelastometry and recombinant factor VIIa in trauma, Curr Opin Anaesthesiol 2009 Apr; 22(2):281â288
45. Hellstern P, Halbmayer WM, Kohler M et al. Prothrombin complex concentrates: indications, contraindications, and risks: a task force summary. Thromb Res 1999; 95:S3âS6.
46. Blauhut B. Indications for prothrombin complex concentrates in massive transfusions. Thromb Res 1999; 95:S63âS69.
47. Schulman S, Bijsterveld NR. Anticoagulants and their reversal. Transfusion Medici- ne Reviews 2007; 21: 37â48.
48. Van Veen SQ, Strengers PF. Use of prothrombin complex concentrates in anticoagulation. Am J Hematol 2008; 83:344â345.
49. Esmon CT. Crosstalk between inflammation and thrombosis.Maturitas. 2004;47:305â314.
50. Rigby AC, Grant MA. Protein S: a conduit between anticoagulation and inflammation. Crit Care Med. 2004;32:S336 âS341
1. Brohi K, Mitchell J, Cohenb, Ross A, Davenporta. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680-685. [ Links ]
2. Charles A, Shaikh AA, Walters M, Huehl S, Pomerantz R. Bloodtransfusion is an independent predictor of mortality after blunttrauma. Am Surg. 2007;73:1-5. [ Links ]
3. Kheirabadi BS, Crissey JM, Deguzman R, Holcomb JB. In vivobleeding time and in vitro thrombelastography measurements are better indicators of dilutional hypothermic coagulopathy thanprothrombin time.J rauma.2007;62:1352-1359 [ Links ]
4. Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography.JThrombHaemost. 2007;5:289 -295. [ Links ]
5. Borgman MA, Spinella PC, Perkins JG, et al. The ratio of bloodproducts transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805- 813. [ Links ]
6. Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a Reassessment. J Trauma. 1995; 38(2):185-193. [ Links ]
7. MacLeod JB, Lynn M, McKenney M, Cohn S, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003; 55(1):39-44. [ Links ]
8. Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J.Trauma.2003;54(6):1127-1130. [ Links ]
9. Stahel PF,Moore EE,Schreier SL, Flierl MA,Kashuk JL. Transfusion Strategies in Posinjury Coagulophaty. Curr Opin Anesth. 2009;22:289-298. [ Links ]
10. Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma.2008; 64:1211-1217. [ Links ]
11. Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg.2007; 245:812-81. [ Links ]
12. Torres JC, Torres MU, Benjumea MA. Manejo de hemorragia digestiva profusa secundaria a hemangioma intestinal con factorVIIa recombinante [Reporte de caso y revisión de la literatura]. Act Colom de Cuid Inten. 2008; 8(4): 322-329. [ Links ]
13. Tanaka, kenichi A, Keil NS, Levy, Jerrold H. Blood Coagulation: Hemostasis and Thrombin Regulation. Anesth. & Analg. 2009 May 108(5) :1433-1446. [ Links ]
14. John RH, Brohi K, Richard PD,Carl JH, John BH , Kluger Y,Mackway K, Par M, , Rizoli SB , Yukioka T , Hoyt DB, Bouillon B. The Coagulopathy of Trauma: A Review of Mechanisms. J Trauma. 2008;65:748 -754. [ Links ]
15. Esmon CT. The interactions between inflammation and coagulation.Bj J Haematol.2005;131:417-430. [ Links ]
16. Mann KG. Biochemistry and physiology of blood coagulation.Thromb Haemost. 1999;82:165-174. [ Links ]
17. Siegel JH, Rivkind AI, Dalal S, et al. Early physiologic predictors of injur Severity and death in blunt multiple trauma. Arch Surg 1990; 125:498-508. [ Links ]
18. Brummel ZK, Whelihan MF, Ziedins EG, Mann KG. Theresuscitative fluid you choose may potentiate bleeding. J Trauma. 2006;61:1350 -1358. [ Links ]
19. Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation?. Am J Surg 2005;190:479-484. [ Links ]
20. Hirshberg A, Dugas M, Banez EI, Scott BG, Wall MJ, Mattox KL. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma 2003;54:454-463. [ Links ]
21. Brazil EV, Coats TJ. Sonoclot coagulation analysis of in-vitro haemodilution with resuscitation solutions. J R Soc Med 2000;93:507-510. [ Links ]
22. Vincent JL, Rossaint R, Riou B, Ozier Y, Zideman D, Spahn DR. Recommendations on the use of rFVIIa as an adjunctive treatment for massive bleeding -a European perspective. Critical Care 2006; 10: R120. [ Links ]
23. Reed RL II, Bracey AW, Jr., Hudson JD, Miller TA, Fischer RP.Hypothermia and blood coagulation: dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990;32:141-152. [ Links ]
24. Wolberg AS, Meng ZH, Monroe DM III, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56:1221-1228. [ Links ]
25. Meng ZH, Wolberg AS, Monroe DM III, Hoffman M. The effect oftemperature and pH on the activity of factor VIIa: implications forthe efficacy of high-dose factor VIIa in hypothermic and acidoticpatients. J Trauma. 2003;55:886-891. [ Links ]
26. Wolberg AS, Meng ZH, Monroe DM, Hoffman MA. systematic evaluation ofthe effect of temperature on coagulation enzyme activity and platelet function.J Trauma 2004; 56:1221-1228. [ Links ]
27. Martini WZ, Pusateri AE, Uscilowicz JM, DelgadoAV, HolcombJB. Independent contributions of hypothermia and acidosis tocoagulopathy in swine. J Trauma. [discussion] 2005;58:1009-1010. [ Links ]
28. Cosgriff N, Moore EE, Sauaia A,Moynihan K, Burch JM,Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. [discussion] 1997;42:857- 861. [ Links ]
29. Martini WZ, Dubick MA, Wade CE,Holcomb JB. Evaluation of tris-hydroxymethyl aminomethane on reversing coagulation abnormalities caused by acidosis in pigs. Crit Care Med. 2007;35:1568 -1574. [ Links ]
30. Levi M, van der Poll T, ten Cate H, van Deventer SJ. The cytokine mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. 1997;27:3-9. [ Links ]
31. Shebuski RJ, Kilgore KS. Role of inflammatory mediators in thrombogenesis. J Pharmacol Exp Ther. 2002;300:729 -735. [ Links ]
32. Chesebro BB, Rahn P, Carles M,Esmon C, Jun X, Brohi K, Frith D, Pittet J-P, Cohen M. Shock. Increase in activated protein c mediates acute traumatic coagulopathy in mice. 2009 Dec ; 32 6:659-665. [ Links ]
33. Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalitiesin trauma patients by rotation thrombelastography. J Thromb Haemost 2007; 5:289-295. [ Links ]
34. Devenport RA, Brohi K. Coagulophaty in trauma patients : Importance of thrombocyte function?.Curr Opin Anesth.2009;22:261-266. [ Links ]
35. Oliver M. Theusinger D R, Ganter M. Transfusion in trauma: why and how should we change our current practice? Curr Opn Anaesth 2009,22:000-000 [ Links ]
36. Feliciano DV, Mattox K, Moore E. Trauma, 6th Edition: McGraw-Hill ;2008. [ Links ]
37. Dutton RP. Current Concepts in Hemorrhagic Shock. Anesth Clinics 2007 March 1; 25 :23-34. [ Links ]
38. American College of Surgeons Committee on Trauma: Advanced Trauma Life Support(ATLS)Course for Physicians,edn 7.Chicago:American College of Surgeons;2008. [ Links ]
39. Sorensen B, Ingerslev J. Tailoring haemostatic treatment to patient requirements - an update on monitoring haemostatic response using thrombelastography. (Suppl) Haemophilia 2005 11; 1:1-6. [ Links ]
40. Brown RB, Klar J, Teres D, Lemeshow S, Sands M. Prospective study of clinical bleeding in intensive care unit patients. Crit Care Med. 1988 Dec 16(12):1171-6. [ Links ]
41. Stein D, Dutton R, Kramer M, Scalea T. Reversal of Coagulopathy in Critically Ill Patients With Traumatic Brain Injury: recombinant Factor VIIa is More Cost-Effective Than Plasma. J Trauma 2009;66: 63-75. [ Links ]
42. Galvan DA,Fink MP. Recombinant factor VIIa in severe trauma: Further study needed. Crit care 2006 10:308. [ Links ]
43. Martinowitz U, Holcomb JB, Pusateri AE, et al. Intravenous rFVIIa administered for hemorrhage control in hypothermic coagulopathic swine ith grade V liver injuries. J Trauma 2001; 50:721-729. [ Links ]
44. Bartal C, Yitzha A. The role of thromboelastometry and recombinant factor VIIa in trauma, Curr Opin Anaesthesiol 2009 Apr; 22(2):281-288 [ Links ]
45. Hellstern P, Halbmayer WM, Kohler M et al. Prothrombin complex concentrates: indications, contraindications, and risks: a task force summary. Thromb Res 1999; 95:S3-S6. [ Links ]
46. Blauhut B. Indications for prothrombin complex concentrates in massive transfusions. Thromb Res 1999; 95:S63-S69. [ Links ]
47. Schulman S, Bijsterveld NR. Anticoagulants and their reversal. Transfusion Medicine Reviews 2007; 21: 37-48. [ Links ]
48. Van Veen SQ, Strengers PF. Use of prothrombin complex concentrates in anticoagulation. Am J Hematol 2008; 83:344-345. [ Links ]
49. Esmon CT. Crosstalk between inflammation and thrombosis.Maturitas. 2004;47:305-314. [ Links ]
50. Rigby AC, Grant MA. Protein S: a conduit between anticoagulation and inflammation. Crit Care Med. 2004;32:S336 -S341 [ Links ]











 text in
text in 

