Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Colombian Journal of Anestesiology
Print version ISSN 0120-3347
Rev. colomb. anestesiol. vol.39 no.1 Bogotá Jan./Mar. 2011
https://doi.org/10.5554/rca.v39i1.160
Artículo de Revisión
Toxicity Due to Local Anesthetic Agents: Literature Review
Rafael Enrico Valencia Gómez*, Hans Fred Garcia Araque**
* Médico, Residente II Ano de Anestesiología, Hospital Militar Central, Bogotá, Colombia, rafael_valencia@hotmail.com
** Médico y Anestesiólogo. Coordinador del Posgrado en Anestesiología, Universidad Militar Central. Comité de Reanimación Sociedad Colombiana de Anestesiología y Reanimación. Coordinador servicio anestesiología, docente pregrado y posgrado U. Militar Nueva Granada, Hospital Militar Central, Bogotá, Colombia, hafregar@gmail.com
Recibido: febrero12 de 2010. Enviado para modificaciones: abril 27 de 2010. Aceptado: agosto 28 de 2010.
SUMMARY
Local anesthetics (LA) are drugs widely used in the practice of anesthesia, with low incidence of adverse events; however, in case of toxicity, mortality is very high. Currently, the systemic toxicity rates have dropped from 0.2 % to 0.01 %, thanks to the use of preventive measures and to the development of safer drugs. In view of the high risk of latent mortality, studies in humans are not feasible and hence the available sources of information are extrapolated animal studies or case reports.
Severe LA toxicity manifestations occur mainly as a result of intravascular administration rather than due to tissue absorption; hence, bupivacaine is the LA that exhibits the highest risk. Clinically there are awareness disorders and tonic-clonic seizures followed by cardiovascular involvement resulting from conduction blocks and difficult to manage cardiovascular collapse.
With regards to management, prevention is the key, followed by an adequate anesthetic technique; rapid identification and diagnosis and early application of ACLS rescue measures. More recently, the concomitant use of 20 % lip-id emulsions has been supported by successful resuscitation case reports.
Keywords: Anesthetics Local, Toxicity, Cardiotoxins, Neurotoxicity Syndromes, Fat Emulsions Intravenous. (Source: MeSH, NLM).
INTRODUCTION
Local anesthetics (LA) are drugs commonly used in anesthesia, particularly in hospitals doing procedures under regional techniques. They are a tool for the treatment or the prevention of acute or chronic pain and for pain management when making a diagnosis or establishing a prognosis (1).
Although the occurrence of adverse events due to LA is infrequent, their study is extremely important because the severity of the toxicity -resulting from the risk of inadequate administration, i.e. high doses, inappropriate site- is associated with a high mortality (2).
Molecular changes result in plasma protein binding properties, lipid solubility (potency), action time, toxicity and ionization (1). Moreover, different molecules may express isomers of varying properties of their racemic molecule (potency, receptor affinity, toxic effects, among others) (3).
Incidence
The available data to this date are supported by low-grade evidence studies since for ethical and obvious reasons, trials with exposure to high levels of toxicity are not allowed in humans. Thus, most of the information comes from extrapolating the results in animal models (4,5). The incidence described by Mulroy for regional procedures is of 200/100,000 cases for brachial plexus block and of 12/ 100,000 for epidural anesthesia (4).
The rates of systemic toxicity due to LA have dropped significantly in the last three decades, from 0.2 % to 0.01 %, due to the application of preventive measures such as aspiration prior to inoculation, the use of trial doses and the establishment of maximum doses, inter alia. The highest toxicity rates have been reported as secondary to the peripheral nerve block (7.5/10,000 cases). The mortality rate was 0.023 cases/100,000 (6). The incidence of regional anesthesia-related seizures was from 1 to 4/1,000 cases with a greater incidence associated with caudal > brachial techniques (supraclavicular - inter-scalene > axillary) > epidural; with regards to LA agents, bupivacaine was the LA with the highest incidence of complications (7-10). (Table 1).
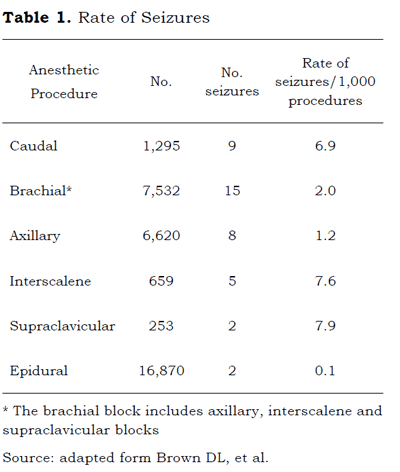
In the study by Barrington et al (11), the incidence of LA toxicity was 0.98/1,000 blocks and the main complications were tonic-clonic seizures, loss of awareness and tachycardia. Minor complications were agitation and signs of mild CNS involvement. There were no events from cardiovascular collapse. Depending on the type of LA, the incidence was higher with ropivacaine and there was one case associated to the use of lidocaine.
PHARMACODYNAMICS AND PHARMACOKINETICS
Voltage-dependent calcium channel blockers, with greater affinity for open channels, is the mode of action for axonal effect that produces a sensorial and motor block. Activity on other types of ion channels (Ca++ and K+) has been described, evidenced by a widening effect, delayed repolarization and changes in the membrane potential that causes a stronger impact on the Na+ channel block (12). The presence of these blockers in the central, cardiac and peripheral spaces explains the symptomatology of AL toxicity (12,13).
Various factors affect the pharmacokinetics of LA agents, including potency (lipophilicity), dose, rate of administration, vascularization of the site of administration for systemic absorption (intravenous > tracheal > intercostal > paracervical > epidural > brachial plexus > ciatic > subcutaneous), metabolism, use of substances that reduce the rate of absorption (epinephrine, bicarbonate, hyaluronidases, etc.) (1,4). The lung has also been described as a protective organ against the toxic effect of the LAs due to the attenuation of blood concentrations (20 %), regardless of the dose (6).
MAXIMUM RECOMMENDED DOSES
The existing information about LAs dosing is mostly not evidence-based; usually it is shown as the maximum total dose for preventing excessive administration and thus toxicity (14). (Table 2).
In this case, the recommendation is to individualize the dose according to the patient's characteristics (age, comorbidities), procedure to be performed, type of LA (pharmacokinetics, pharmacodynamics), etc. (14,15). In the case of amino esters LAs, these are matabolized by plasma estearases and reduce the risk of acute toxicity; however, the opposite is true with regards to their metabolites that are responsible for anaphylactic reactions (5).
Among the clinical uses of LAs, the tumescent techniques used in plastic surgery for liposuction (16) use maximum accepted doses of li-docaine with epinephrine - 35 mg/kg and up to 55 mg/kg have been reported - with serum levels not exceeding 5 ug/mL. In any case, it is important to keep in mind the potential occurrence of LA-related toxicity, regardless of the dose or resulting from drug interactions.
TOXICITY RESULTING FROM LOCAL ANESTHETICS
Originally described by Mayer in 1928, who published 40 cases of deaths following the administration of local anesthetic techniques (17); recently, Dr. Albright reported the risk of the new LAs in 1979 (17). Later, increasing importance has been given to the pathophysi-ological and therapeutic description of acute toxicity caused by LA agents.
LA systemic reactions are rare and their incidence tends to decrease due to the positive affect of established procedures and safer LAs. Despite its low incidence, the outcomes can be fatal. Systemic reactions usually present themselves with mild manifestations and are frequently unnoticed, leading to a probable under-registration of such events (6).
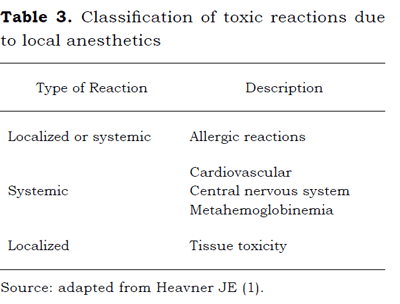
The acute toxic effects following the intravascular administration of LAs tend to be more severe and short lasting than those arising from the slow systemic absorption of perineural administration. This is due to the by-pass of the body's clearance systems (1). Usually these effects occur as a result of the inadvertent intravascular or intrathecal injection, secondary to high doses. There is mainly initial CNS involvement - greater susceptibility -and subsequent CV collapse (3,4). Three types can be differentiated, depending on the reaction (Table 3).
Long lasting LAs, despite the current availability of isomers developed (levobupivacaine, ropivacaine), with a view to reduce the toxic effects of bupivacaine, are still particularly important, due to the presence of severe acute toxicity events (18-21).
LOCAL TOXICITY
Miotoxicity: LAs may cause tissue necrosis at the site of administration and two of the causes for this complication are trauma and bleeding resulting from the adminsitration of the anesthetic agent. Laboratory studies have described a higher risk of tissue toxicity with the use of bupivacaine > procaine > tetracaine > ropivacaine (22). A condrotoxic effect has been experimentally described with the intraarticular administration of bupivacaine (23).
Neurotoxicity: neurotoxicity has been shown at high concentrations in laboratory models; however, it may not be attributed exclusively to LAs, since other types of substances should be considered as well; i.e. preservatives (22). Likewise, transient neurological signs have been described (disesthesias, parestesias, decreased or temporary loss of motor function, incontinence, Cauda Equina syndrome, etc.). these signs are not permanent and are resolved within one to four weeks (23). Experimental studies have shown a greater tendency of lidocaine to cause neurotoxicity of spinal anesthesia (2 to 5 % above other LAs) (22).
SYSTEMIC TOXICITY
Central Nervous System toxicity: with the progressive increase in LAs serum concentration following their administration, there is an increased risk of central toxicity due to its greater sensitivity. Initially there is a depressor effect on the inhibitory pathways mediated by the GABA receptors (22), and a stimulating effect over the NMDA receptors (22), with clinical manifestations such as agitation, dizziness, myoclonic responses, nistag-mus, disartria, muscle contractures, perioral parestesias, taste disorders (metallic taste), hearing disorders (tinnitus), poor response to verbal instructions, speech disorders and finally, tonic-clonic seizures (22,23). At higher serum concentrations, the depressor effect of the inhibitory pathways continues and blocks the excitatory pathways, causing respiratory depression (hypoxia, acidosis) and coma (22,23).
Cardiovascular toxicity: Acute hemodynamic changes are the result of a systemic response to acute toxicity that generates changes in the cardiovascular (direct effect) and central nervous system (indirect effect mediated by the stimulus to the autonomic nervous system) (6). Cardiac toxicity is expressed through two mechanisms: one, associated with autonomic ganglion dysfunction and second, as an effect over the miocardial conduction systems. Ventricular arrhythmias are a rare occurrence, except with bupivacaine, that has shown tachycardia and ventricular fibrilation (24).
Studies in animal models (25-29) have established the direct effect of LAs on the cardiac cell, evidenced by a widening of the QRS, disruption of contractility and malignant arrhythmias leading to cardiac failure from the disruption of modulation mediated by ion channels G proteins (Ca++ y K+), mitochondrial metabolism and ATP production (30). Among the various LAs and based on animal models, bupi-vacaine has shown the greatest cardiotoxicity due to the strong attraction and dissociation of the sodium channels, as well as to the effects on the slow calcium channels (10).
The serum concentrations required for AL severe toxicity to occur (except for bupivacaine) in the heart, exceed those required to produce tonic-clonic seizures; the clinical manifestations are tachicardia, hypertension (initially), hypotension and bradycardia (severe toxicity), miocardial depression and low cardiac output associated with arrhythmias (delayed conduction, branch blocks, PR prolongation, ventricular ectopia, ventricular tachycardia, ventricular fibrilation, torsade de pointes, sinus arrest, asystole) (22,30,31). Likewise, the ischemic cardiac disease and conduction disorders encourage LA related toxicity (17). Table 4 shows the serum concentrations of various LAs at the time of cardiovascular collapse, in accordance with an animal model study.
THERAPEUTIC APPROACH
It must be said that every procedure with regional techniques should be performed under the appropriate conditions to prevent the occurrence of complications. Consequently, basic, non-invasive monitoring should be provided (pulse oximetry, continuous electrocardiographic monitoring and non-invasive arterial pressure control, keeping in mind the cardiovascular complications and the need to identify them early on to start corrective actions). Additionally, the steps to prevent overdosing, aspiration prior to infiltration, test dose and dose fragmentation should be implemented. All these measures have decreased the occurrence of adverse events during the last few decades (7-9).
Overdosing Prevention: It is clear that having the necessary resources available to respond to an LA acute toxicity event could improve the outcome (17). Therefore, all the necessary inputs should be at hand for an adequate management of the airway; i.e., selecting the best LA and the optimum dose and concentration (22), choosing the most favorable technique and the appropriate anatomic approach. It must also be kept in mind that the use of bupivacaine results in a higher depressor effect over the cardiac conduction, especially in the ventricular space. Likewise, the association of bupivacaine-lidocaine is related with a higher increase in the number of cardiac conduction disorders due to the additive effect of lidocaine (24,27-29).
Pretreatment: The use of benzodiazepines in animal models (17) reduces the likelihood of seizures due to CNS toxicity, although the sedation could delay the early diagnosis in case of AL acute toxicity.
Trial dose: The objective of a trial dose is the early identification of intravascular injection (17), taking into account several factors that may alter the result (age, gestation, use of drugs such as benzodiazepines, b-blockers, opiates, clonidine, general anesthesia) (17,32,33).
The side effect of administering 15 mcg of epi-nephrine (increased HR > 10 x/min, SBP > 15 mmHg or 25 % drop in the T wave amplitude in DII) (17); a low output may alter this result.
Dose Fractioning: fractioning the dose administered (slow dose 5 mL, with frequent aspirations and non-invasive monitoring (34)), will reduce the maximum arterial concentration, although there is no experimental evidence yet as to the impact of the result. Early detection of any toxic effects to stop the administration of the LA in case of suggestive symptoms, although is not enough to prevent an acute LA toxicity, does provide the time required to reduce the blood concentrations and allow for distribution and removal (6).
Early Response: Early response to the identification of mild symptoms of toxicity increases the probability of success with treatment (30,31). In the presence of suggestive symptoms, the necessary therapeutic measures should be initiated (securing the airway and adequate ventilation; administration of resuscitation maneuvers in accordance with ACLS protocols, prevention and early treatment of seizures, correcting arrhythmias and myocar-dial depression) (30,31).
The administration of epinephrine at high doses (animal models) during the resuscitation maneuvers, together with Lipid Emulsions (LE) for managing cardiovascular toxicity due to bupivacaine, showed no benefits in recovery and suggests that this combination will prevent the reversal of bupivacaine toxicity (35). Hiller et al (36) showed the effect of high doses of epinephrine on the concomitant use of LE, possibly resulting from increased acidosis due to higher lactate and showed better effects al doses below 10 mcg/Kg (36).
Cardiopulmonary bypass or the use of LE (18) should also be considered in case of toxicity refractory to treatment (30,31). It must be emphasized that propofol does not replace this type of drugs because of its low lipid content and cardiodepressant effect.
USE OF LIPID EMULSIONS
There are different theories about the mechanism of action of the intravenous administration of LEs. Among the most widely accepted theory is the serum extraction from the LAs through a lipid interface; others treat the metabolic effect by reestablishing the delivery of fatty acids to the mitochondria in order to restore the energy output. The activation of the calcium and potassium channels has also been shown, as well as the decrease of tissue acidosis and in the production of CO2 during ischemic episodes associated with cardiovascular collapse (10).
The solubility of long lasting LAs in Lipid Emulsions and their high binding potential, probably explains their clinical efficacy in the management of LA toxicity. Among these LEs, long chain triglycerides are more effective than the medium chain triglycerides, as shown by Mazoit et al (17). Moreover, the pharmacokinetic properties of the LAs (hydrophobicity), favor this effect, while acidosis reduces the binding potential. Zausig et al (37) suggest that the effects of LEs on cardiovascular toxicity (cardiac arrest) induced by the LAs, is strongly dependent on lipophilicity of each particular type of anesthetic and the best effects are from bupivacaine induced toxicity. However, additional studies are required to validate these results.
The overall acceptance of lipid emulsions as a potential antidote for the treatment of LA toxicity (38) has generated the interest of medical associations to include them in guidelines (39), protocols and recommendations in various printed and e-media (31); (http://www.lipidrescue.org, http://www.asahq.org/clinical/Anesthesiology-CentricACLS.pdf, http://www.resus.org.uk/pages/caLocalA.htm). According to these publications, LEs have an acceptable safety profile at the doses recommended by the AAGBI (Association of Anesthetists of Great Britain and Ireland) (31). Similarly, around 2008, the ASA Critical Care Medical Committee, as well as the UK Resuscitation Council, made on line publications of protocols for the management of LA toxicity.
Cave et al (40) did a study to assess the guidelines published by AAGBI for the management of LA toxicity with a view to assess their efficacy. This protocol showed a trend towards the spontaneous and faster recovery of the circulation versus the control group. Although these findings are encouraging, additional experimental evidence is required.
Overall, the case reports published have shown the effectiveness of 20 % LEs (41,42), in diverse patient conditions, including the successful utilization in obstetric patients (obstetric analgesia with peridural bupivacaine) (43); pediatric patients (caudal anesthesia with bupivacaine) (44); and in other cases where LEs are recommended to reduce the progression of acute toxicity after the onset of mild symptoms (17,42,45-51). Moreover, LEs are a useful tool to revert LA toxicity, in addition to other previous advanced vital cardiovascular support measures that result in limited response (52).
ADVERSE EFFECTS
Despite the use of appropriate doses and good tolerability in accordance with previous reports, the most frequent adverse effects are related to administration, systemic inflammatory response, allergic reactions, anaphylactic reactions, head ache, somnolence, dizziness, diaforesis, disnea, nausea, vomiting, hyperthermia and hypercoagulability (10).
Late effects are thrombocytopenia, jaundice, volume overload, elevated transaminases, leucopenia, warfarin resistance (protein binding), interference with the membrane oxygenation loops, hepato-splenomegaly, pancreatitis (53), fatty embolism (particles > 5 u), pulmonary embolism -the last two related with the dose and infusion rate-; increased intracranial pressure following severe cranioencephalic trauma (54); risk of toxicity relapse following administration and predisposition to seizures in children (55).
CONTRAINDICATIONS
The presence of lipid disorders, egg allergy, acute myocardial infarction. It is also recommended to use with caution in cases of anemia, severe liver disease, coagulopathies, lung disease and patients at risk for fatty embolism (10).
AL acute toxicity research in humans is not possible for ethical reasons. The studies (understanding the pharmacological, pathophysi-ological and therapeutic mechanisms) completed thus far are based on animal models subsequently applied and extrapolated to humans. Due to the ethical limitations to determine the ideal LE dosis for the treatment of LA toxicity, these doses have yet to be determined.
CONCLUSIONS
Although a rare occurrence, LA toxicity should be an important consideration due to the mortality impact as a result of severe reactions such as cardiovascular collapse. Consequently, early identification based on the clinic and a high suspicion index is mandatory, in addition to the on-going application of various preventive measures to lower the risk of occurrence.
Additionally, the concomitant use of LEs based on the recommendations from successful case reports, should also be considered for resuscitation based on ACLS protocols.
REFERENCES
1. Heavner JE. Local Anesthetics, Curr Opin Anesthesiol, 2007, 20336-342.
2. Veering BT. Complications and local anaesthetic toxicity in regional anaesthesia. Curr Opin Anaesth, 2003;16:455-9.
3. Leone S, Di Cianni S, Casati A, et al. Pharmacology, toxicology and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed, 2008;79:92-105.
4. Mulroy MF. Sistemic toxicity and cardiotoxicity from local anesthetics: incidence and preventive measures. Regional Anesthesia and Pain Medicine, 2002;27(6):556-561
5. Reynolds F. Maximum Recommended Doses of Local Anesthetics: A Constant Cause of Confusion. To the editor. Regional Anesthesia and Pain Medicine. 2005; 30(3):314-6.
6. Mather LE, Copeland SE, Ladd LA. Acute Toxicity of Local Anesthetics: Underlying Pharmacokinetic and Pharmacodynamic Concepts. Reg Anesth Pain Med 2005;30:553-66.
7. Brown DL, Ransom DM, Hall JA, et al. Regional Anesthesia and Local Anesthetic-Induced Systemic Toxicity: Seizure Frequency and Accompanying Cardiovascular Change. Anesth Analg 1995;81:321-8.
8. Cox B, Duriex ME, Marcus MA. Toxicity of local anaesthetics. Best Pract Res Clin Anaesthesiol. 2003 Mar;17(1):111-36
9. Faccenda KA, Finucane BT. Complications of regional anaesthesia, incidence and prevention. Drug Saf. 2001;24(6):413-4.
10. Felice KL, Schumann HM. Intravenous Lipid Emulsion for Local Anesthetic Toxicity: A Review of the Literature. J Med Toxicol, 2008;4(3):184-91.
11. Barrington MJ, Watts SA, Gledhill SR, et al. Preliminary Results of the Australasian Regional Anaesthesia Collaboration A Prospective Audit of More Than 7000 Peripheral Nerve and Plexus Blocks for Neurologic and Other Complications. Reg Anesth Pain Med 2009;34:534-41.
12. Kindler CH, Yost CE. Two-pore domain potassium channels: new sites of local anesthetic action and toxicity. Reg Anesth Pain Med 2005;30:260-274.
13. Hollmann MW, Herroeder S, Kurz KS, et al. Time-dependent inhibition of G-protein coupled receptor signaling by local anesthetics. Anesthesiology. 2004;100:852-860.
14. Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: A multi-factorial concept. Reg Anesth Pain Med 2004;29:564-575.
15. Niesel HC. Local anesthetics maximum recommended doses. Anaesthesiol Reanim. 1997;22(3):60-2.
16. Kucera IJ, Lambert TJ, Klein JA, et al. Liposuction: contemporary issues for the anesthesiologist. Journal of Clinical Anesthesia 2006;18:379-87.
17. Weinberg GL. Lipid Infusion Therapy: Translation to Clinical Practice, Anesth Analg 2008;106:5 1340-42.
18. Mazoit J, Le Guen R, Beloeil H, et al. Binding of Long-lasting Local Anesthetics to Lipid Emulsions, Anesthesiology 2009; 110:380-6.
19. Foxall G, McCahon R, Lamb J, et al. Levobupivacaine-induced seizures and cardiovascular collapse treated with Intralipid. Anaesthesia. 2007;62:516-518.
20. Chazalon P, Tourtier JP, Villevielle T, et al. Ropivacaine-induced Cardiac Arrest after Peripheral Nerve Block: Successful Resuscitation. Anesthesiology 2003; 99:1449-51.
21. Huet O, Eyrolle LJ, Mazoit JX, et al. Cardiac Arrest after Injection of Ropivacaine for Posterior Lumbar Plexus Blockade Anesthesiology 2003; 99:1451-3.
22. Tasch MD, Butterworth JF. Toxicity of local anesthetics, ASA REFRESHING COURSE, 2006.
23. Zink W, Graf BM. The toxicity of local anesthetics: the place of ropivacaine and levobupivacaine. Current Opinion in Anaesthesiology. 2008;21:645-650.
24. Harmatz A. Local Anesthetics: Uses and Toxicities. Surg Clin N Am 2009;89:587-98.
25. Heavner JE. Cardiac toxicity of local anesthetics in the intact isolated heart model: a review. Reg Anesth Pain Med 2002;27:545-555.
26. Liu P, Feldmahn S, Covino BM, et al. Acute cardiovascular toxicity of intravenous amide local anesthetics in anesthetized ventilated dogs. Anesth Analg 1982;61:317-22.
27. Simon L, Kariya N, Pelle-Lancien E, et al. Bupivacaine-Induced QRS Prolongation is Enhanced by Lidocaine and by Phenytoin in Rabbit Hearts. Anesth Analg 2002;94:203-7.
28. Mets B, Janicki PK, James MF, et al. Lidocaine and Bupivacaine Cardiorespiratory Toxicity Is Additive: A Study in Rats. Anesth Analg 1992;75:611-4.
29. Thomas RD, Behbehani MM, Coyle DE, et al. Cardiovascular Toxicity of Local Anesthetics: An Alternative Hypothesis. Anesth Analg 1986;65:444-50.
30. Weinberg GL. Current Concepts in Resuscitation of Patients With Local Anesthetic Cardiac Toxicity. Reg Anesth Pain Med. 2002; 27(6): 568-75.
31. The Association of Anaesthetists of Great Britain & Ireland 2007. Guidelines for the Management of Severe Local Anaesthetic Toxicity. http://www.aagbi.org/publications/guidelines/docs/latoxicity07.pdf. 2007.
32. Copeland SE, Ladd LA, Gu X, et al. The Effects of General Anesthesia on Whole Body and Regional Pharmacokinetics of Local Anesthetics at Toxic Doses. Anesth Analg 2008;106:1440-9.
33. Copeland SE, Ladd LA, Gu X, et al. The Effects of General Anesthesia on the Central Nervous and Cardiovascular System Toxicity of Local Anesthetics. Anesth Analg 2008;106:1429-39.
34. Corcoran W, Butterworth J, Weller RS, et al. Local Anesthetic-Induced Cardiac Toxicity: A Survey of Contemporary Practice Strategies Among Academic Anesthesiology Departments. Anesth Analg 2006;103:1322-26.
35. Hicks SD, Salcido DD, Logue ES, et al. Lipid emulsion combined with epinephrine and vasopressin does not improve survival in a swine model of bupivacai-neinduced cardiac arrest. Anesthesiology 2009;111:138-46.
36. Hiller DB, Di Gregorio G, Ripper R, et al. Epinephrine Impairs Lipid Resuscitation from Bupivacaine Overdose A Threshold Effect. Anesthesiology 2009;111:498-505.
37. Zausig YA, Zink W, Keil M, et al. Lipid Emulsion Improves Recovery from Bupivacaine-Induced Cardiac Arrest, but Not from Ropivacaine- or Mepivacaine-Induced Cardiac Arrest. Anesth Analg 2009;109:1323-6.
38. Picard J, Ward SC, Zumpe R, et al. Guidelines and the adoption of "lipid rescue" therapy for local anaesthetic toxicity. Anaesthesia 2009; 64:122-125.
39. Picard J, Harrop-Griffiths W. Lipid emulsion to treat drug overdose: past, present and future. Anaesthesia 2009; 64:119-121.
40. Cave G, Harvey MG, Winterbottom T. Evaluation of the Association of Anaesthetists of Great Britain and Ireland lipid infusion protocol in bupivacaine induced cardiac arrest in rabbits. Anesthesia. 2009;64(7):732-7.
41. Leskiw U, Weinberg GL. Lipid resuscitation for local anesthetic toxicity: is it really lifesaving? Curr Opin Anaesthesiol 2009;22:667-71.
42. Weinberg G. Lipid infusion resuscitation for local anesthetic toxicity: Proof of clinical efficacy. Anesthesiology 2006; 105:7-8.
43. Spence A. Lipid reversal of central nervous system symptoms of bupivacaine toxicity. Anesthesiology 2007; 107:516-517.
44. Shah S, Gopalakrishnan S, Apuya J, et al. Use of Intralipid in an infant with impending cardiovascular collapse due to local anesthetic toxicity Anesth 2009:23:439-41.
45. Rosenblatt MA, Abel M, Fischer GW, et al. Successful Use of a 20% Lipid Emulsion to Resuscitate a Patient after a Presumed Bupivacaine-related Cardiac Arrest. Anesthesiology 2006;105:217-8.
46. Ludot H, Tharin JY, Belouadah M, et al. Successful Resuscitation After Ropivacaine and Lidocaine-Induced Ventricular Arrhythmia Following Posterior Lumbar Plexus Block in a Child. Anesth Analg 2008;106:1572-4.
47. Warren JA, Thoma RB, Georgescu A, et al. Intravenous Lipid Infusion in the Successful Resuscitation of Local Anesthetic-Induced Cardiovascular Collapse After Supraclavicular Brachial Plexus Block. Anesth Analg 2008;106:1578-80.
48. Gregorio GD, Schawartz D, Ripper R, et al. Lipid emulsion is superior to vasopressin in a rodent model of resuscitation from toxin-induced cardiac arrest. Crit Care Med 2009; 37:993-999.
49. Picard J, Ward SC, Zumpe R, et al. Guidelines and the adoption of 'lipid rescue' therapy for local anaesthetic toxicity. Anaesthesia. 2009 Feb;64(2):122-5.
50. Litz RJ, Popp M, Stehr SN, Koch T. Successful resuscitation of a patient with ropivacaine-induced asystole after axillary plexus block using lipid infusion. Anaesthesia 2006;61:800-1.
51. Litz R, Roessel T, Heller AR, et al. Reversal of Central Nervous System and Cardiac Toxicity After Local Anesthetic Intoxication by Lipid Emulsion Injection. Anesth Analg 2008;106:1575-7.
52. Corman SL, Skledar SJ. Use of Lipid Emulsion to Reverse Local Anesthetic-Induced Toxicity Corman and Skledar. Ann Pharmacother 2007;41:1873-7.
53. Weinberg GL. Limits to Lipid in the Literature and Lab: What We Know, What We Don't Know. Anesth Analg 2009;108(4):1062-4.
54. Brull SJ. Lipid Emulsion for the Treatment of Local Anesthetic Toxicity: Patient Safety Implications. Anesth Analg. 2008;106:1337-8.
55. Marwick PC, Levin AI, Coetzee AR. Recurrence of Cardiotoxicity After Lipid Rescue from Bupivacaine-Induced Cardiac Arrest. Anesth Analg 2009;108: 1344-6.
Conflicto de intereses: ninguno declarado
1. Heavner JE. Local Anesthetics, Curr Opin Anesthesiol, 2007, 20336-342. [ Links ]
2. Veering BT. Complications and local anaesthetic toxicity in regional anaesthesia. Curr Opin Anaesth, 2003;16:455-9. [ Links ]
3. Leone S, Di Cianni S, Casati A, et al. Pharmacology, toxicology and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed, 2008;79:92-105. [ Links ]
4. Mulroy MF. Sistemic toxicity and cardiotoxicity from local anesthetics: incidence and preventive measures. Regional Anesthesia and Pain Medicine, 2002;27(6):556-561 [ Links ]
5. Reynolds F. Maximum Recommended Doses of Local Anesthetics: A Constant Cause of Confusion. To the editor. Regional Anesthesia and Pain Medicine. 2005; 30(3):314-6. [ Links ]
6. Mather LE, Copeland SE, Ladd LA. Acute Toxicity of Local Anesthetics: Underlying Pharmacokinetic and Pharmacodynamic Concepts. Reg Anesth Pain Med 2005;30:553-66. [ Links ]
7. Brown DL, Ransom DM, Hall JA, et al. Regional Anesthesia and Local Anesthetic-Induced Systemic Toxicity: Seizure Frequency and Accompanying Cardiovascular Change. Anesth Analg 1995;81:321-8. [ Links ]
8. Cox B, Duriex ME, Marcus MA. Toxicity of local anaesthetics. Best Pract Res Clin Anaesthesiol. 2003 Mar;17(1):111-36 [ Links ]
9. Faccenda KA, Finucane BT. Complications of regional anaesthesia, incidence and prevention. Drug Saf. 2001;24(6):413-4. [ Links ]
10. Felice KL, Schumann HM. Intravenous Lipid Emulsion for Local Anesthetic Toxicity: A Review of the Literature. J Med Toxicol, 2008;4(3):184-91. [ Links ]
11. Barrington MJ, Watts SA, Gledhill SR, et al. Preliminary Results of the Australasian Regional Anaesthesia Collaboration A Prospective Audit of More Than 7000 Peripheral Nerve and Plexus Blocks for Neurologic and Other Complications. Reg Anesth Pain Med 2009;34:534-41. [ Links ]
12. Kindler CH, Yost CE. Two-pore domain potassium channels: new sites of local anesthetic action and toxicity. Reg Anesth Pain Med 2005;30:260-274. [ Links ]
13. Hollmann MW, Herroeder S, Kurz KS, et al. Time-dependent inhibition of G-protein coupled receptor signaling by local anesthetics. Anesthesiology. 2004; 100:852-860. [ Links ]
14. Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: A multi-factorial concept. Reg Anesth Pain Med 2004;29:564-575. [ Links ]
15. Niesel HC. Local anesthetics maximum recommended doses. Anaesthesiol Reanim. 1997;22(3):60-2. [ Links ]
16. Kucera IJ, Lambert TJ, Klein JA, et al. Liposuction: contemporary issues for the anesthesiologist. Journal of Clinical Anesthesia 2006;18:379-87. [ Links ]
17. Weinberg GL. Lipid Infusion Therapy: Translation to Clinical Practice, Anesth Analg 2008;106:5 1340-42. [ Links ]
18. Mazoit J, Le Guen R, Beloeil H, et al. Binding of Long-lasting Local Anesthetics to Lipid Emulsions, Anesthesiology 2009; 110:380-6. [ Links ]
19. Foxall G, McCahon R, Lamb J, et al. Levobupivacaine-induced seizures and cardiovascular collapse treated with Intralipid. Anaesthesia. 2007;62:516-518. [ Links ]
20. Chazalon P, Tourtier JP, Villevielle T, et al. Ropiva-caine-induced Cardiac Arrest after Peripheral Nerve Block: Successful Resuscitation. Anesthesiology 2003; 99:1449-51. [ Links ]
21. Huet O, Eyrolle LJ, Mazoit JX, et al. Cardiac Arrest after Injection of Ropivacaine for Posterior Lumbar Plexus Blockade Anesthesiology 2003; 99:1451-3. [ Links ]
22. Tasch MD, Butterworth JF. Toxicity of local anesthetics, ASA REFRESHING COURSE, 2006. [ Links ]
23. Zink W, Graf BM. The toxicity of local anesthetics: the place of ropivacaine and levobupivacaine. Current Opinion in Anaesthesiology. 2008;21:645-650. [ Links ]
24. Harmatz A. Local Anesthetics: Uses and Toxicities. Surg Clin N Am 2009;89:587-98. [ Links ]
25. Heavner JE. Cardiac toxicity of local anesthetics in the intact isolated heart model: a review. Reg Anesth Pain Med 2002;27:545-555. [ Links ]
26. Liu P, Feldmahn S, Covino BM, et al. Acute cardiovascular toxicity of intravenous amide local anesthetics in anesthetized ventilated dogs. Anesth Analg 1982;61:317-22. [ Links ]
27. Simon L, Kariya N, Pelle-Lancien E, et al. Bupivacaine-Induced QRS Prolongation is Enhanced by Lidocaine and by Phenytoin in Rabbit Hearts. Anesth Analg 2002;94:203-7. [ Links ]
28. Mets B, Janicki PK, James MF, et al. Lidocaine and Bupivacaine Cardiorespiratory Toxicity Is Additive: A Study in Rats. Anesth Analg 1992;75:611-4. [ Links ]
29. Thomas RD, Behbehani MM, Coyle DE, et al. Cardiovascular Toxicity of Local Anesthetics: An Alternative Hypothesis. Anesth Analg 1986;65:444-50. [ Links ]
30. Weinberg GL. Current Concepts in Resuscitation of Patients With Local Anesthetic Cardiac Toxicity. Reg Anesth Pain Med. 2002; 27(6): 568-75. [ Links ]
31. The Association of Anaesthetists of Great Britain & Ireland 2007. Guidelines for the Management of Severe Local Anaesthetic Toxicity. http://www.aagbi.org/publications/guidelines/docs/latoxicity07.pdf. 2007. [ Links ]
32. Copeland SE, Ladd LA, Gu X, et al. The Effects of General Anesthesia on Whole Body and Regional Pharmacokinetics of Local Anesthetics at Toxic Doses. Anesth Analg 2008;106:1440-9. [ Links ]
33. Copeland SE, Ladd LA, Gu X, et al. The Effects of General Anesthesia on the Central Nervous and Cardiovascular System Toxicity of Local Anesthetics. Anesth Analg 2008;106:1429-39. [ Links ]
34. Corcoran W, Butterworth J, Weller RS, et al. Local Anesthetic-Induced Cardiac Toxicity: A Survey of Contemporary Practice Strategies Among Academic Anesthesiology Departments. Anesth Analg 2006;103:1322-26. [ Links ]
35. Hicks SD, Salcido DD, Logue ES, et al. Lipid emulsion combined with epinephrine and vasopressin does not improve survival in a swine model of bupivacai-neinduced cardiac arrest. Anesthesiology 2009;111:138-46. [ Links ]
36. Hiller DB, Di Gregorio G, Ripper R, et al. Epinephrine Impairs Lipid Resuscitation from Bupivacaine Overdose A Threshold Effect. Anesthesiology 2009;111:498-505. [ Links ]
37. Zausig YA, Zink W, Keil M, et al. Lipid Emulsion Improves Recovery from Bupivacaine-Induced Cardiac Arrest, but Not from Ropivacaine- or Mepivacaine-Induced Cardiac Arrest. Anesth Analg 2009;109:1323-6. [ Links ]
38. Picard J, Ward SC, Zumpe R, et al. Guidelines and the adoption of "lipid rescue" therapy for local anaesthetic toxicity. Anaesthesia 2009; 64:122-125. [ Links ]
39. Picard J, Harrop-Griffiths W. Lipid emulsion to treat drug overdose: past, present and future. Anaesthesia 2009; 64:119-121. [ Links ]
40. Cave G, Harvey MG, Winterbottom T. Evaluation of the Association of Anaesthetists of Great Britain and Ireland lipid infusion protocol in bupivacaine induced cardiac arrest in rabbits. Anesthesia. 2009;64(7):732-7. [ Links ]
41. Leskiw U , Weinberg GL. Lipid resuscitation for local anesthetic toxicity: is it really lifesaving?. Curr Opin Anaesthesio. 2009;22:667-71. [ Links ]
42. Weinberg G. Lipid infusion resuscitation for local anesthetic toxicity: Proof of clinical efficacy. Anesthesiology 2006; 105:7-8. [ Links ]
43. Spence A. Lipid reversal of central nervous system symptoms of bupivacaine toxicity. Anesthesiology 2007; 107:516-517. [ Links ]
44. Shah S, Gopalakrishnan S, Apuya J, et al. Use of Intralipid in an infant with impending cardiovascular collapse due to local anesthetic toxicity Anesth 2009:23:439-41. [ Links ]
45. Rosenblatt MA, Abel M, Fischer GW, et al. Successful Use of a 20% Lipid Emulsion to Resuscitate a Patient after a Presumed Bupivacaine-related Cardiac Arrest. Anesthesiology 2006;105:217-8. [ Links ]
46. Ludot H, Tharin JY, Belouadah M, et al. Successful Resuscitation After Ropivacaine and Lidocaine-Induced Ventricular Arrhythmia Following Posterior Lumbar Plexus Block in a Child. Anesth Analg 2008;106:1572-4. [ Links ]
47. Warren JA, Thoma RB, Georgescu A, et al. Intravenous Lipid Infusion in the Successful Resuscitation of Local Anesthetic-Induced Cardiovascular Collapse After Supraclavicular Brachial Plexus Block. Anesth Analg 2008;106:1578-80. [ Links ]
48. Gregorio GD, Schawartz D, Ripper R, et al. Lipid emulsion is superior to vasopressin in a rodent model of resuscitation from toxin-induced cardiac arrest. Crit Care Med 2009; 37:993-999. [ Links ]
49. Picard J, Ward SC, Zumpe R, et al. Guidelines and the adoption of 'lipid rescue' therapy for local anaesthetic toxicity. Anaesthesia. 2009 Feb;64(2):122-5. [ Links ]
50. Litz RJ, Popp M, Stehr SN, Koch T. Successful resuscitation of a patient with ropivacaine-induced asystole after axillary plexus block using lipid infusion. Anaesthesia 2006;61:800-1. [ Links ]
51. Litz R, Roessel T, Heller AR, et al. Reversal of Central Nervous System and Cardiac Toxicity After Local Anesthetic Intoxication by Lipid Emulsion Injection. Anesth Analg 2008;106:1575-7. [ Links ]
52. Corman SL, Skledar SJ. Use of Lipid Emulsion to Reverse Local Anesthetic-Induced Toxicity Corman and Skledar. Ann Pharmacother 2007;41:1873-7. [ Links ]
53. Weinberg GL. Limits to Lipid in the Literature and Lab: What We Know, What We Don't Know. Anesth Analg 2009;108(4):1062-4. [ Links ]
54. Brull SJ. Lipid Emulsion for the Treatment of Local Anesthetic Toxicity: Patient Safety Implications. Anesth Analg. 2008;106:1337-8. [ Links ]
55. Marwick PC, Levin AI, Coetzee AR. Recurrence of Cardiotoxicity After Lipid Rescue from Bupivacaine-Induced Cardiac Arrest. Anesth Analg 2009;108: 1344-6. [ Links ]











 text in
text in 

