Introduction
Cardiac arrest is a public health problem, as survival rates remain very low despite early access to advanced paramedical and emergency care, and improved treatment.1 The frequency of extracorporeal membrane oxygenation (ECMO) utilization in adults has increased over recent years2,3; consequently, we will see an increased number of urgent noncardiac surgeries in adults on ECMO. Venoarterial ECMO (VA-ECMO) is an adaptation of conventional cardiopulmonary bypass techniques utilized for prolonged cardiopulmonary support and can be implemented by intrathoracic or extrathoracic cannulation.4 ECMO is a proven rescue strategy for patients with cardiac arrest who are unresponsive to conventional cardiopulmonary resus-citation.5 It offers a bridge in patients with refractory cardiac arrhythmias who respond poorly to defibrillation and anti-arrhythmic medications.6-8
We discuss the case of a patient on VA-ECMO who underwent multiple urgent noncardiac surgeries, and we propose several issues to consider for the perioperative management of patients on ECMO. Written consent was obtained.
Case
A 56-year-old, male (height 168 cm, mass 79kg) suffered out-of-hospital cardiac arrest and was defibrillated once due to ventricular fibrillation (VF) by a bystander with successful return of spontaneous circulation (ROSC). The electrocardiogram showed a large anterior ST segment elevation myocardial infarction. He was taken straight to the catheterization laboratory where a percutaneous balloon angioplasty was performed to an occluded left anterior descending artery and an intraortic balloon pump (IABP) was inserted. His ejection fraction on ventriculogram was 30%. There were signs of triple-vessel disease and he was taken for emergency coronary artery bypass graft. He was transferred to the intensive care unit (ICU). Postoperative recovery was unremarkable, the patient was extubated, and the IABP was removed, and he was alert, oriented, and without any clear neurological deficits. He was transferred to the floor 3 days later, and he then developed rapid atrial fibrillation with a ventricular rate as high as 200, which was treated with metoprolol and amiodarone. He subsequently developed VF, and ROSC was attained following short-duration cardiopulmonary resuscitation (CPR). The patient was then transferred to the ICU, intubated and sedated.
The patient developed recurrent defibrillation-resistant episodes of VF and was defibrillated approximately 20 to 25 times. He received numerous amiodarone boluses. He was started on a lidocaine infusion and a bolus of procainamide. An IABP was reinserted at the bedside. There were no acute ST segment changes when he was in sinus rhythm and cardiac enzymes were dropping. There was no evidence of preexcitation. Transthoracic and transoesophageal echocardiography (TEE) was performed at the bedside, revealing no new regional motion abnormality. Electrophysiology considered that the arrhythmogenicity was derived from injured myocardium and not a new acute infarct. Ventricular ablation was carried out in the hopes of managing the rhythm and avoiding heart transplantation if we could get his rhythm managed. At first, the patient seemed to do well off antiarrhythmics, as he was gradually woken up. Unfortunately, he went into another intractable electrical storm with ventricular tachycardia (VT) and polymorphic VF, refractory to cardioversion and defibrillation attempts. CPR was given for an entire hour and it was clear that he would require mechanical circulatory support to survive. The patient's neurological and end-organ status as well as the appropriateness of heart transplantation were to be assessed in the following days. The family was apprised of the situation. During ongoing cardiopulmonary resuscitation, and having obtained family consent, the patient was peripherally cannulated for VA-ECMO, and was later switched to central cannulation.
On the second postoperative day, following VA-ECMO placement, a significant hemoglobin loss prompted focused abdominal sonographic assessment and abdominal computed tomography (CT), scan revealing splenic lacerations with arterial extravasation thought to be secondary to vigorous chest compressions given on the day of ECMO insertion. Subsequently, endovascular embolization of the proximal splenic artery failed; transfusion of packed red blood cells (PRBCs), fresh frozen plasma, and emergent splenectomy were required.
Preoperatively, electrolytes, coagulation studies, and fibrinogen were normal, with the exception of mild thrombocytopenia. Vital signs were reassuring: invasive blood pressure (BP) 130/70, heart rate (HR) 90beats/min, oxygen saturation 98%, sinus rhythm. Arterial and central venous cannulas remained from the previous surgery, as did the endotracheal tube. Vasopressors and inotropes were not required. After transfer to the operating theater, standard monitors and external defibrillation pads were placed, and general anesthesia and analgesia were initiated and titrated to bispectral index.
Cardiac rhythm remained stable throughout the intervention; as the last stitches were placed, the patient showed bursts of VT despite normal electrolyte and blood gas results. Lidocaine and procainamide were given but, ultimately, multiple defibrillations were required. Mean arterial pressures were maintained at 50 to 80 mm Hg throughout the case by effective VA-ECMO with flow rates remaining at 4 to 5l/min, despite nonperfusing rhythm. The surgical intervention lasted 1hour, and transfer to the ICU was uneventful.
The following day, in view of refractory VT and persisting bleeding, a repeat CT scan revealed a large hematoma in the splenic bed, with ongoing extravasation. Transfusion of 2 units of PRBC and return to the operating theater for exploration were indicated; TEE was indicated for assessment of myocardial function and to exclude intracavitary thrombus. On exploration, a large splenic bed hematoma was evacuated, followed by packing of the left upper quadrant. TEE showed moderate left ventricular dilation, global severe left and right ventricular dysfunction, septal dyskinesis, well-positioned ECMO cannulae, and a small pericardial effusion. The surgical time was short and the patient was returned again to intensive care.
Subsequent surgical interventions were required to remove abdominal packing, close the wound and, later, to control an omental bleeding. Heparinized for ECMO, the patient was kept on infusion until 4 hours before surgery. Cardiac rhythm was stable with only premature ventricular contractions. The patient returned to intensive care after each intervention. Furthermore, the patient ultimately underwent cardiac transplantation and survived to discharge from hospital.
Discussion
VA-ECMO support is being used increasingly in the adult population to provide temporary mechanical cardiorespiratory support.7,9 The management of these patients necessarily involves a multidisciplinary team; it is even recommended to have an echocardiography-trained physician on the team.7 Coordination within the multi-disciplinary team for transfer to and from the operating theater is essential.
Noncardiac procedures in patients on ECMO are usually emergent or urgent procedures. These patients are more susceptible to bleeding and pericardial collections because of systemic anticoagulation,7 and any invasive procedure is associated with an increased risk of mortality secondary to anticoagulation.10 Consequently, it is necessary to confirm availability of blood products, use blood conservation strategies such as cell salvage, and have an excellent intravenous access.
Clotting screen, complete blood count, electrolytes, and optimized volume status are required before invasive procedures.10 Goals must include an activated clotting time of 160 to 180 seconds, platelets >100,000/mm, and fibrinogen 150 to 300mg/dl.10
External defibrillation pads are generally accepted as mandatory for use in patients with a high likelihood of unstable arrhythmia. This includes their use in the intensive care, during transfers, and while in the operating suite. Ideally, the role of charging and discharging the device remains assigned to a particular individual at all times, in order to avoid confusion and improve care coordination during crises. However, patients on ECMO may better tolerate VT and VF, making it an indication as rescue therapy in electrical storm.
VA-ECMO provides systemic and myocardial perfusion by delivery of oxygenated blood to the aorta. However, any blood escaping the venous cannula may traverse the lung, which should be ventilated and oxygenated with a conservative, lung protective strategy. Failure to oxygenate this blood may result in deoxygenated blood being ejected into the aorta and subsequently pushed into the coronary arteries, or head vessels, depending on myocardial performance at that time. Ideally, ventilatory settings should be kept the same as in the ICU, the goal being to keep low peak airway pressure (<35cmH2O), plateau pressure under 25 cmH2O, respiratory rate of 10 breaths/minute, positive end-expiratory pressure under 10cmH20, and FiO2 under 0.4.10 ECMO allows a reduction in tidal volume in order to limit plateau pressures to a lung ultra protective range of <6 ml/ kg predicted body weight (PBW) or even <4 ml/kg PBW.11
The aforementioned settings will be different in the acute respiratory distress syndrome arena.
Echocardiographic examinations are not routine for noncardiac cases but, if they are used, it is important to rule out the presence of an intracannula thrombus affecting the blood flow at the orifice of the outflow cannula.7,12 This can be assessed in 2D, with color and pulsed wave Doppler interrogation.7,12 More generally, monitoring and assessment of cardiac chamber size, intermittent aortic valve opening, and ruling out intracavitary thrombi in low cardiac output states is essential. It is necessary to rule out aortic insufficiency and guide fluid responsiveness. Importantly, TEE warrants additional care due to the likelihood of repeated examinations in the anti-coagulated patient; the most experienced operator should insert the probe and avoid unnecessary probe manipulation. Video laryngoscopy-guided probe may facilitate a more gentle insertion in patients with significant edema or risk of bleeding.13
ECMO cannulas can limit exposure of the abdominal or thoracic cavity and it is essential for the surgical team to secure the lines, ensuring no catastrophic cannula displacement or vessel disruption. Table 1 summarizes some of the perioperative strategies used in VA-ECMO.14
Table 1 Perioperative strategies used on VA-ECMO
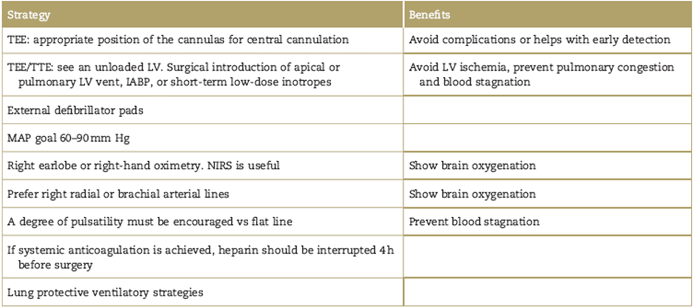
TEE=transoesophageal echocardiogram; TTE=transthoracic echocardiogram; LV=left ventricle; IABP=intra-aortic ballon pump; MAP=mean arterial pressure; NIRS=near-infrared spectroscopy.
Source: Authors.
Patient transfer requires a sedate, immobile patient; sedation and neuromuscular blockade may be required. Depending on the anticipated length of transfer, consideration is given to availability of additional oxygen tanks, ECMO pump battery or power source, manual cranks, circuit components, and blood products.10,15 Although uncommon, complications such as power failure, circuit tubing leakage, circuit rupture, oxygenator membrane thrombosis, hypercapnia, and hypoventilation have all been reported.10,15
The use of ECMO in adults has grown in recent years and there is no doubt that an increasing number of urgent noncardiac surgeries will be performed in this adult population. This case illustrates some of the many challenges that may occur/recur in the care of this patient population and our approach to addressing these risks.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no personal patient identifiers appear in this article.











 text in
text in 


