Introduction
Ascites is abnormal fluid accumulation in the peritoneal cavity1. In the Western world, cirrhosis is responsible for ascites in 80% of cases2; it is the most common complication of cirrhosis, occurring in up to 5%-10% of patients with cirrhosis each year3. Other etiologies include cancer (10%), heart failure (5%), and tuberculosis, among others4. Within this last group, uroperitoneum stands out, the incidence of which is unknown; there are only case reports and series in the literature. The leading cause of uroperitoneum is traumatic bladder rupture, which represents 96% of all cases. Another 3% is due to increased bladder volume in conditions with altered sensitivity, such as neurogenic and postpartum bladder with epidural anesthesia, and less than 1% is due to spontaneous rupture5. Additionally, a mortality rate of up to 47% has been documented, mainly due to septic shock if not diagnosed promptly5.
In the initial study of ascites, the analysis of the peritoneal fluid and the calculation of the serum ascites albumin gradient (SAAG)6 are essential. SAAG is calculated by subtracting the concentration of serum albumin and the peritoneal fluid; when greater than 1.1 g/dL, it allows the diagnosis of ascites secondary to portal hypertension with an accuracy of 97%, sensitivity of 93% and specificity of 47%7,8. However, sometimes it is impossible to establish a diagnosis with this initial approach, and the study must be deepened4,9. Other studies that should be performed on ascitic fluid based on clinical suspicion are total protein, cytochemical, cultures, amylase, pH, and adenosine deaminase, among others4. Therefore, it is sometimes necessary to determine the gradient between ascitic fluid creatinine and serum creatinine in case of suspicion of uroperitoneum, which supports this diagnosis if greater than 1.010-12 both in cirrhotic and non-cirrhotic patients since it has been shown that the electrolytes, urea, and creatinine values in the ascitic fluid are similar to those in the serum13,14. We present the case of a patient with uroperitoneum due to a defect in the bladder dome who presented with abdominal pain and recurrent tension ascites associated with acute renal pseudo-injury with a fluctuating elevation of nitrogen gases.
Case presentation
A 68-year-old man with a history of prostate carcinoma underwent a radical prostatectomy in 2016. As a complication of this procedure, there was a lesion in the right distal ureter that required ureterovesical reimplantation. Since 2019, he has had three hospitalizations for recurrent ascites that required evacuating paracentesis on several occasions. The ascitic fluid study showed a SAAG greater than 1.1 mg/dL during these hospitalizations, suggesting portal hypertension. The most common etiologies of ascites, including chronic liver disease, heart failure, malignancy, tuberculosis, autoimmunity, and thrombosis, were ruled out. Given the diagnostic uncertainty and imaging findings of particulate fluid with inflammatory characteristics, diagnostic laparoscopy was performed twice with a biopsy of the peritoneum and omentum, which revealed mature adipose tissue with chronic inflammatory infiltrate and reactive mesothelial cells with no evidence of neoplasia. In addition, the serum creatinine value was variable, mg/dL at times and, after permanent bladder catheterization, 0.8 mg/dL without any signs of obstructive compromise of the urinary tract on renal ultrasound.
He was admitted to our institution due to a fourth episode of ascites and abdominal pain. On admission, he had a blood pressure of 126/88 mm Hg, a heart rate of 100 beats per minute (bpm), and was afebrile. Physical examination revealed a distended but soft abdomen, a positive ascitic wave, mild pain in the hypogastrium, and no masses, hepatomegaly, or splenomegaly.
The diagnostic process began again; liver disease and thrombosis of the splanchnic axis were ruled out. Diagnostic and therapeutic paracentesis was performed, draining 3,600 mL of sallow fluid. SAAG was greater than 1.1 mg/dL. Proteins in the ascitic fluid were 1.58 g/dL, although particulate ascitic fluid was striking in the abdominal ultrasound, suggestive of infectious or inflammatory involvement. Microbiological cultures were negative, and other paraclinical tests were requested, as described in Table 1.
Table 1 Laboratory parameters on admission to the institution
| Laboratory parameters | Results |
|---|---|
| Hemoglobin | 16.1 g/dL |
| Hematocrit | 45.9% |
| Leukocytes | 7,740 cells per μL |
| Neutrophils | 4,820 cells per μL |
| Lymphocytes | 1,810 cells per μL |
| Platelets | 339,000 cells per μL |
| ALT | 16 UI/L |
| AST | 18 UI/L |
| AP | 153 UI/L |
| GGT | 17 UI/L |
| Total bilirubin | 1.24 mg/dL |
| Direct bilirubin | 0.43 mg/dL |
| Albumin | 4.06 g/dL |
| Sodium | 140 mEq/L |
| Potassium | 4.27 mEq/L |
| Chlorine | 100 mEq/L |
| Phosphorus | 4.46 mEq/L |
| Calcium | 9.44 mEq/L |
| Creatinine | 1.7mg/dL |
| BUN | 30 mg/dL |
| Total serum protein | 6.77 g/dL |
| Serum albumin | 4.06 g/dL |
| Albumin in ascitic fluid | 1.1 g/dL |
| SAAG | 2.96 g/dL |
| Proteins in ascitic fluid | 1.58 g/dL |
| Creatinine in ascitic fluid | 7.44 mg/dL |
| Urea in ascitic fluid | 144 mg/dL |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; AP: alkaline phosphatase; SAAG: serum ascites albumin gradient; GGT: γ-glutamyl transferase. Source: The authors.
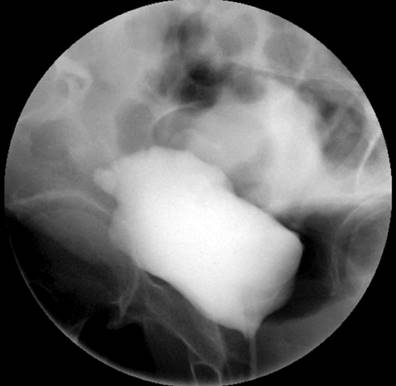
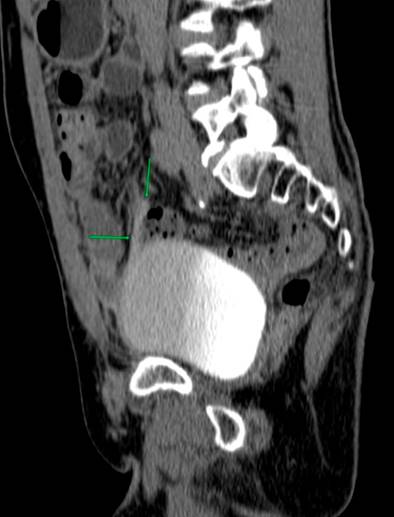
Due to the recurrence of the symptoms, chronicity, absence of etiology after a detailed study, and taking into account the patient’s surgical history, less frequent etiologies were considered a possibility of uroperitoneum. Creatinine was measured in the peritoneal fluid, the result of which was 7.44 mg/dL, and the gradient between fluid and serum was 5.74, which further increased the suspicion. Due to the uncertainty in the face of acute kidney injury and suspicion of urinary fistula, the nephrology service requested a voiding cystography with dynamic films, finding a bladder dome defect with active contrast extravasation into the peritoneal cavity, which confirmed the presence of uroperitoneum and explained the elevation of nitrogenous gases that simulated acute renal injury (Figure 1). To better characterize the genitourinary tract lesion and surgical planning, a urotomography was performed (Figure 2) with evidence of a defect in the bladder dome of 7.8 mm towards the right side in its anterior portion, with evidence of extraluminization of the contrast medium as a sign of rupture. After this characterization, he was scheduled for laparotomy with cystorrhaphy, performed at another institution.
Discussion
Uroperitoneum or urinary ascites is a rare entity defined as the presence of urine in the peritoneal cavity. It may be due to trauma, spontaneous rupture of the bladder, or perforation10. Moreover, it has been iatrogenically described as secondary to surgical procedures such as gastrointestinal15, urological16,17, gynecological18, and obstetric19 surgeries with an incidence of 0.11%20. The main risk factors identified for iatrogenic bladder injury are advanced age, recent chemotherapy or radiotherapy, and smoking20. It has also been associated with neurological conditions such as tabes dorsalis and multiple sclerosis21,22. There are reports in the literature related to radiotherapy and pelvic nerve fibrosis leading to the neurogenic bladder23 and ureterovesical lesions resulting from lithiasis, tumors, and inflammation24. Spontaneous bladder rupture may be due to continuous irrigation, postpartum, and alcoholic intoxication12,24,25. There are three types of bladder rupture: extraperitoneal, the most frequent manifestation, in approximately 80% of cases; intraperitoneal, in up to 15%-20%; and combined26.
The clinical manifestations of urinary peritonitis are not specific since sterile urine can be tolerated for several days and manifest through diffuse symptoms such as abdominal pain, dysuria, frequency, and urgency. There may also be oliguria or anuria and, in severe cases, peritonitis and septic shock if infected urine is present27.
Pseudorenal failure is defined as elevated serum creatinine, which mimics acute kidney injury, often of unknown etiology, after excluding traditional causes28. The uroperitoneum is characterized by increased serum creatinine due to the reabsorption of urinary creatinine mediated by the peritoneal membrane. In turn, chronic intra-abdominal urine leakage increases the abnormal reabsorption of toxic metabolites; thus, consideration should be given to this entity in the presence of acute kidney injury together with ascites or peritonitis, and electrolyte disturbances such as hyponatremia, hyperkalemia, and metabolic acidosis10,27,29. Calculating the gradient between creatinine in ascitic fluid and serum creatinine is instrumental, which gives a higher value to the diagnostic assumption if greater than 110-12, as happened in the case of our patient, whose result was 5.74.
Uroperitoneum is a difficult diagnosis, especially without a history of trauma or urologic instrumentation. Its form of manifestation is variable. It can occur as an acute abdomen with high associated mortality rates or as oliguric pseudo-acute kidney injury accompanied by azotemia, hyponatremia, hyperkalemia, metabolic acidosis, and increased nitrogen levels in the peritoneal fluid. Renal function usually improves when performing paracentesis or indwelling bladder catheterization10,27,30-32.
Voiding cystography is the gold standard for diagnosing bladder rupture, with a sensitivity and specificity of 95% and 100%, respectively33. However, with the availability of new techniques, CT cystography has a performance comparable to voiding cystography, especially if injuries to other organs must be ruled out, which is common in traumatic bladder ruptures in multiple trauma patients33,34.
Sam Kant et al.10 described the case of a 74-year-old man with a history of ischemic heart disease and radical prostatectomy, who ten days after the procedure, presented with oliguric acute kidney injury, ascites, hyponatremia, hyperkalemia, elevated serum urea, and creatinine in the peritoneal fluid. Therefore, they suspected that the source of the abdominal fluid was a urinary focus, like the case described, except for the initial hydroelectrolytic disorder.
Ajape AA et al.35 reported the case of a 62-year-old man with a history of prostatic hyperplasia who consulted for symptoms of abdominal pain, distension, and anuria of five days’ evolution. His abdomen was distended and painful in the hypogastrium. Rectal examination revealed an enlarged prostate and abdominal ultrasound showed free fluid. All of this gave rise to the suspicion of a spontaneous bladder rupture confirmed by a voiding cystography. This case had a manifestation similar to the one we reported: pain, abdominal distension, and confirmation of the uroperitoneum with voiding cystography.
Bourgeois S et al.26 informed the case of a 64-year-old woman with a history of transitional cell carcinoma of the bladder with subsequent laparoscopic nephroureterectomy. She was admitted due to acute colicky abdominal pain, abdominal distension, tender abdomen on palpation, and signs of peritonitis. From the paraclinical tests, a serum creatinine of 4.23 mg/dL and urea of 99.9 mg/dL stood out, with no hydroelectrolytic disorder. A simple tomography showed hypodensity compatible with the free intraperitoneal fluid, suggesting ascites. A prerenal acute kidney injury was suspected, and intravenous fluids were started without a subsequent drop in creatinine until bladder catheterization was performed. A diagnostic laparoscopy was performed, and biopsies were taken from the bladder due to its history of previous neoplasia, which was negative for malignancy. Then, when analyzing the ascitic fluid, elevated creatinine, and urea levels were found (although they were not described), thus confirming the uroperitoneum. This picture is similar to the one we report regarding clinical manifestations, the diagnostic process, the elevation of nitrogen compounds in the peritoneal fluid for the diagnostic approach, and the improvement in renal function after the bladder catheter insertion.
Furthermore, Gonzalo L et al.32 detailed the case of a patient with a history of hypothyroidism and two deliveries, one of them with forceps and bladder rupture, who developed a picture of pain in the hypogastrium and accumulation of peritoneal fluid. A blood urea nitrogen (BUN) of 36 mg/dL and creatinine of 2.16 mg/dL stand out among the initial laboratory parameters. Subsequently, a non-inflammatory peritoneal fluid and a SAAG greater than 1.1 g/dL without liver disease were studied, for which a cystoscopy was performed, finding a vesicoperitoneal fistula, compatible with uroperitoneum, a complex diagnosis as in the case of our patient. Unlike this case, our patient’s symptoms had a larvate and recurrent manifestation, with higher elevations of nitrogen gases.
It should be noted that the different series share the presence of hyperkalemia, increased BUN and creatinine, and improvement of nitrogen levels with permanent bladder catheterization, in addition to increased creatinine and BUN in the peritoneal fluid.
In the present case, the chronicity, the chronological order of the symptoms, their recurrence, the fluctuation in serum creatinine, and the sensible diagnostic approach adopted by our institution added to the history of radical prostatectomy allowed us to suspect a uroperitoneum first with subsequent imaging confirmation. Finally, the patient underwent urology surgery with repair of the bladder defect and has not had recurrences of the clinical picture.











 text in
text in