Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Odontología Universidad de Antioquia
Print version ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.26 no.1 Medellín July/Dec. 2014
REVIEW ARTICLE
THE ROLE OF NOTCH SIGNALING PATHWAY IN THE DEVELOPMENT OF CRANIOFACIAL STRUCTURES
Belfran Alcides Carbonell Medina1
1 Dentist, higher education specialist, magister in Dentistry, Instituto de Genética, Universidad Nacional de Colombia. Email: bacarbonellm@unal.edu.co
SUBMITTED: JANUARY 22/2012-ACCEPTED: NOVEMBER 19/2013
Carbonell BA. The role of NOTCH signaling pathway in the development of craniofacial structures. Rev Fac Odontol Univ Antioq 2014; 26(1): 164-179.
ABSTRACT
The NOTCH signaling pathway is a cell-cell signaling mechanism evolutionarily conserved among species, which is essential for proper embryonic development as it participates in a variety of cellular processes such as proliferation, differentiation, apoptosis, epithelial- mesenchymal transformation, migration, angiogenesis, stem cell maintenance, and cell fate determination. Several genes of this pathway have been implicated in the development of craniofacial structures. 80% of Alagille syndrome patients have mutations in the gene that codes for receptor Jagged1 (Jag1), along with midface hypoplasia and sporadic craniosynostosis. Mice with gene Jagged2 (Jag2) homozygous mutations present cleft palate as a result of ectopic fusions between the tongue and palatal processes. Similarly, mutations induced in the Hes1 gene produce developmental defects in craniofacial structures resulting from cranial neural crest cells (CrnNC), including cleft palate, frontal bone agenesis, cranial base malformation, and reduced size of upper and lower maxilla. Recent studies have shown alterations during tooth morphogenesis in Jagged2-/- mutant mice, accompanied by defects in ameloblasts cytodifferentiation and poor enamel matrix deposition. These studies show that NOTCH signaling pathway is involved in the development of a variety of craniofacial structures such as palate, teeth, maxillaries, and skull. The purpose of this article is to review the different functions of NOTCH signaling during the development of these craniofacial structures and the alterations resulting from mutations in some NOTCH signaling genes such as Jagged2, Jagged1, Hes1,Notch1, and Notch2.
Key words: NOTCH pathway, craniofacial development, palatogenesis, Jagged1.
INTRODUCTION
The craniofacial complex is formed by a set of structures including, roughly, the viscerocranium (face) and the neurocranium.1 Craniofacial development is perhaps one of the most complex processes during embryogenesis since it requires a wide variety of interactions among different embryonic tissues.2, 3 The tissues that produce craniofacial structures in vertebrates originate from ectoderm, mesoderm, endoderm, and cranial neural crest cells (CrnNC).3, 4 During embryogenesis, signals from the ectoderm and the endoderm reciprocally contribute to regulate cell processes such as proliferation, survival, migration, and differentiation of facial mesenchyme, through epithelial-mesenchymal interactions.5, 6 The facial mesenchyme contains cranial neural crest cells (CrnNC) as well as cells of the cephalic paraxial mesoderm.7
The cephalic paraxial mesoderm produces voluntary facial muscle and endothelial cells, whereas CrnNC produce craniofacial connective tissue, such as dentin, dental pulp, facial cartilage, viscerocranium bones, and the anterior region of the skull base.3, 5, 7 Several signaling pathways have been associated to the development of the craniofacial complex, some of the most studied are BMP (Bone Morphogenetic Protein), SHH (Sonic Hedgehog), WNT (Wingless), TGF-beta (Transforming Growth Factor Beta), FGF (Fibroblast Growth Factor), and the NOTCH signaling pathway has been recently associated to it.8-11 The NOTCH signaling pathway is involved in the embryonic and postnatal stages in several cell processes such as proliferation, differentiation, apoptosis, maintenance of undifferentiated stem cells, and cell fate differenciation.12 Mutations induced in several genes of the NOTCH signaling pathway such as Jagged2 and Hes1 in animal models like mice have shown alterations in the development of various craniofacial structures including palate, teeth, jaws, cranial base, and cranial vault.13-15
The most commonly reported craniofacial alterations include cleft palate, morphological alterations and poor formation of dentin matrix, frontal bone agenesis, decreased maxillaries size, and premature closure of cranial fissures, generating a craniosynostosis phenotype with the participation of the Twist1 gene.10, 13-15 In addition, 70 to 80% of Alagille Syndrome patients report mutations in the Jagged1 gene, which encodes a ligand belonging to the NOTCH pathway.16 This syndrome is characterized by multi-organic and craniofacial alterations such as wide forehead, pointed chin, bulbous nose tip, inverted triangle facial appearance, and occasional craniosynostosis.17-19 The objective of this article was to review the role of NOTCH signaling pathway components, such asNotch1, Notch2, Jagged1, Jagged2, and Hes1, in the development of craniofacial structures like palate, teeth, skull, and maxillaries.
An overview of the NOTCH signaling pathway
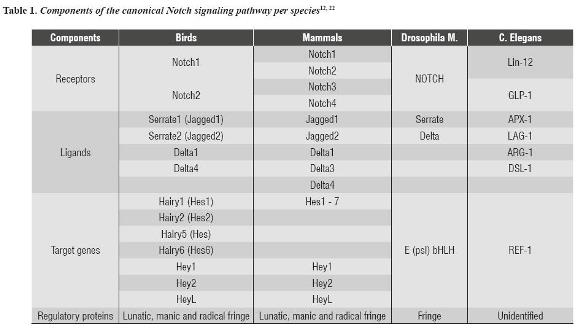
Canonical NOTCH signaling pathway is an evolutionarily conserved mechanism of cell-cell signaling, which participates in a variety of cell processes such as cell fate specification, proliferation, apoptosis, adhesion, epithelial-mesenchymal transformation, migration, angiogenesis, stem cells maintenance, and homeostasis of adult tissues.12, 20, 21 Canonical NOTCH signaling pathway contains several components, including NOTCH receptors, ligands (DSL), target genes (genes of the bHLH, Hes and Hey family), and other regulatory proteins of this pathway as described table 1.
In mammals, like humans and mice, four NOTCH receptors (Notch1, Notch2, Notch3, and Notch4), five ligands (Jag1, Jag2, Delta1, Delta3, and Delta4) and several target genes have been described; some of the most studied are Hes1, Hes5, and Hey1.23 The NOTCH pathway receptor is a transmembrane protein that receives signals of transmembrane ligands which are expressed in neighboring cells.22
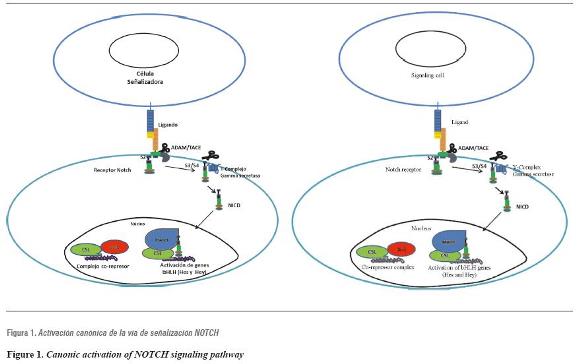
Direct contact of receptor and ligand triggers a series of proteolytic events at the level of the NOTCH receptor, causing the receptor's intracellular domain to translocate to the nucleus where it activates transcription of target genes Hes and Hey (figure 1).22 The signals transduced by these receptors play a pivotal role in various stages of embryonic development.24 Deficiencies and abnormal growth of NOTCH signaling have been significantly associated with developmental anomalies and cancer.18, 25-28
Contact between the ligand of a signaling cell (at the top) and the NOTCH receptor of a neighboring cell (at the bottom) produces a series of proteolytic events at the extracellular and intracellular domains of the NOTCH receptor, causing the NOTCH intracellular domain (NICD) to translocate to the nucleus, activating the transcription of genes of the bHLH, Hes and Hey family through interaction with CSL transcription factor and the MAML co-activator.
The NOTCH pathway in the development of secondary palate
The development of secondary palate takes place around stage E12-E15 in mice, corresponding to the period between the 8th and 12th week of gestation in humans.27 During this period, the palatal processes generated at the internal edges of the maxillary prominences grow vertically on both sides of the tongue, then they elevate and position horizontally on the dorsum of tongue, to finally contact each another and merge, producing a split between the oral cavity and the nasal cavity.28 Each of these palatogenesis steps are highly regulated by several signaling pathways, and any failure during growth, lifting, contact or fusion of the palatal processes produces cleft palate.27
The expression of several NOTCH signaling pathway genes has been detected during normal palate development.10 Genes that encode for Notch2 and Notch3 receptors are expressed in the lingual mesenchyme and in mandibular, maxillary and palatal processes from E12.5 to E14.5. Contrary to this pattern of mesenchymal expression, Jag2 andNotch1 are co-expressed mainly in the lingual, maxillary and palatine epithelium and in the mandibular lateral epithelium.10 Homozygous mice with Jag2-/- mutations show cleft palate accompanied by ectopic fusions between the dorsal region of the tongue and the palatal processes.13 The cleft palate phenotype in these mutants is attributed to such ectopic fusions, which inhibit proper elevation of the palatal processes and therefore generate cleft palate.10, 13
Pathological palate-tongue fusions have been described in humans.29, 30 However, the cellular and molecular mechanisms involved in this pathology are not totally clear. In 2006, Casey identified in a mouse high levels of theNotch1 receptor activated during the differentiation process of cells of the lingual, palatal, maxillary and mandibular periderm, from E11.5 to E14.5.10 Mutant Jag2sm/sm homozygous mice, in addition to cleft palate and palate-tongue fusions, show aberrant fusions between the maxillary and mandibular processes accompanied by reduction ofNotch1 activated levels during the formation of oral periderm, with disorganization and loss of the flat morphology of the periderm cells that cover the palate and tongue.10
This suggests that Jag2 is the ligand responsible for activation ofNotch1 and oral periderm differentiation. Therefore the Jag2-Notch1 signaling appears to be temporally and spatially regulated during early palate development, to prevent premature adhesions between palatal processes and the rest of the oral epithelium in contact, and thus facilitate lifting, contact, adhesion and fusion of palatal processes.10 In addition, other authors have shown that NOTCH signaling pathway, mediated by Jag2 and IRF6 (transcription interferon regulatory factor 6) function as signaling pathways converging during the process of oral epithelium differentiation during palatogenesis.31
Another NOTCH signaling pathway gene involved in the development of craniofacial structures like palate is gene Hes1. 15 Mice with homozygous mutations Hes1-/- show alterations during secondary palatogenesis, characterized by poor growth, premature lifting, and horizontal reorientation of palatal ridges, which generates cleft palate since the small size of palatal ridges thwarts their contacting and merging in most mutants.15 In addition, these mutant mice show defects in cranial base development associated with agenesis of the sphenoid bone. The specific cellular and molecular mechanisms involved in these mutants' phenotype are still unknown. However, the authors suggest that early differentiation of cranial neural crest cells with reduced proliferation may have induced premature elevation and inadequate size of the palatal ridges. 15
NOTCH signaling pathway during odontogenesis: Jagged2 regulates tooth morphogenesis and differentiation
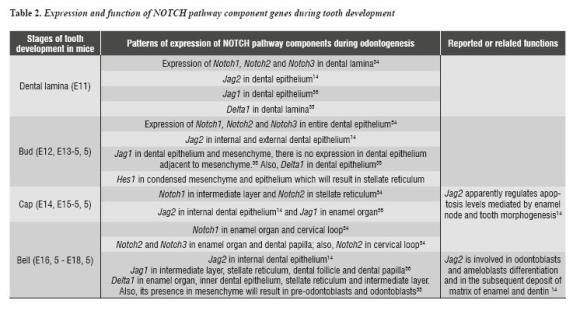
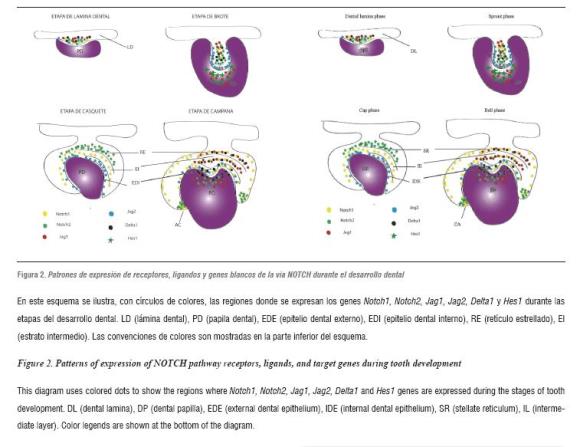
In addition to NOTCH pathway participation in the development of structures like palate, various components of this signaling pathway have been identified during tooth development in mice. Several studies have shown that components of the NOTCH signaling pathway are expressed during tooth development in mice. The expression ofNotch1, Notch2, Notch332 , Dll133 , Jag134 and Jag235, 36 prefigure ameloblastic and non-ameloblastic region subdivision in the initial stages of tooth development (table 2). This is evident during the cytodifferentiation stage, in which several NOTCH receptors and some of their ligands show complementary expression patterns:Notch1 expression is limited to the intermediate layer, while Dll1 and Jag2 are expressed in the layer adjacent to the inner dental epithelium (figura 2).33, 35, 36
Similarly, in the dental mesenchyme, Dll1 is expressed in odontoblasts in process of differentiation, while NOTCH genes are expressed in the sub-odontoblastic layer mainly.34 These findings suggest that receptors and ligands of the NOTCH pathway participate in the processes of cellular differentiation during tooth development. Recent studies have examined the expression, regulation, and function of Jag2 gen in tooth development in mice.14 These studies have shown that Jag2 is expressed in epithelial cells, enabling enamel production (ameloblasts) during the early stages of tooth development.
Likewise, tissue recombination experiments have shown that Jag2 expression in the epithelium is regulated by signals from the mesenchyme.14 In vitro cultures of dental epithelium explants show that local application of FGF stimulates Jag2 expression, while the application of BMP produces the opposite effect. This suggests that during tooth development, the Jag2 gene expression is controlled by FGF in mesenchyme and by BMP from mesenchyme. Homozygous Jag2 mutant mice show a variety of dental anomalies.14 Their molars' crown morphology is deformed, with additional cusps, and their incisors ameloblast cytodifferentiation is inhibited as well as enamel matrix deposition.
These results demonstrate that the NOTCH pathway, mediated by Jag2, is essential for correct odontogenesis.14, 37, 38 Recent studies have shown a new function of the NOTCH pathway mediated by theNotch1 receptor and one of its Hes1 effector proteins in the development of cervical loop. Using a NOTCH pathway inhibitor known as DAPT on in vitro and in vivo mouse models demonstrated that blocking the Hes1 gene expression resulted in an increase in apoptosis levels and a decrease in proliferation of stem cells from the cervical loop.39 These findings suggest that, by means of Hes1, the NOTCH pathway controls survival of epithelial stem cells of the developing cervical loop.39
Involvement of the NOTCH pathway in the craniofacial phenotype of patients with Alagille Syndrome and Hajdu-Cheney Syndrome
Alagille Syndrome is an autosomal dominant disorder that affects the hepatic, cardiac, skeletal, kidney, and eye systems as well as facial development. 17, 40 Alagille Syndrome is caused by haploinsufficiency of gen Jagged1 mainly.16, 19, 41 However, gene Notch2 mutations have been identified in a subgroup of patients with this syndrome.18, 42, 43 Alagille Syndrome is characterized by craniofacial abnormalities including wide forehead, pointed chin, bulbous nose tip, and midfacial third hypoplasia, producing an inverted triangle facial appearance of occasional craniosynostosis.17-19, 40, 44 The typical inverted V face feature is found in 95% of diagnosed patients, based on the intrabiliary hepatic duct phenotype.40, 45 These facial features suggest that Jagged1 is involved in midface morphogenesis.
Several animals like zebrafish and mice have been used to model Alagille Syndrome characteristics, including the craniofacial ones.46-48 Mice models have allowed identifying the role of Jagged1 during facial morphogenesis. Deletion of Jagged1 in cranial neural crest cells using a Wnt1- cre; Jag1 Flox/Flox mouse allowed recapitulating the midface hypoplasia phenotype in Alagille Syndrome patients.49 The etiology of midface hypoplasia in these mice was a consequence of a reduced proliferation of CrnNC in the midface, aberrant vasculogenesis, and poor production of extracellular matrix in palatal processes, associated with abnormal growth in the facial area.49 Decreased maxilla size and poor elongation of palatal processes were also evident.
Regarding cranial vault development, Jagged1 has been involved in the undifferentiated maintenance of immature pre-ontogenic cells during development of cranial sutures, ensuring a harmonious development of brain growth and sutures closing in mouse models.50 These studies have shown that Jagged1 works as a target gene of the TWIST1 transcription factor to regulate the expression of genes such as Β-catenin, Smad 1/3/8 and PrK1/2, involved in cranial vault osteoblast differentiation.50 Hence Jagged1 maintains osteoblastic precursor cells undifferentiated and therefore it keeps cranial sutures. Then, Jagged1 mutations result in premature closure of coronal sutures, generating a craniosynostosis phenotype, as sporadically observed in Alagille Syndrome.44, 50
Another syndrome with alterations in craniofacial structures development is the Hajdu-Cheney.51, 52 This syndrome has autosomal dominant inheritance, although sporadic cases can also be found. Patients with this syndrome have a point mutation in exon 34 of gene Notch2, which produces a defect in the PEST domain synthesis of the intracellular region of protein Notch2.53 The PEST domain is involved in the ubiquitination and degradation of the NOTCH receptor's intracellular portion; therefore, absence of this domain impedes the proper regulation of NOTCH pathway activation.22 The craniofacial phenotype of Hajdu-Cheney syndrome patients includes the following features: facial dimorphism, micrognathism, poor closure of cranial sutures, and wormian bones formation.51 The role of Notch2 gene during craniofacial structures development has not been established to date. Therefore, it is necessary to study animal models to analyze the cellular and molecular mechanisms that are altered during development of the craniofacial structures affected in this syndrome.
CONCLUSIONS
Current studies on NOTCH pathway's genes alterations and their involvement in craniofacial structures development, such as palate, cranial vault, cranial base, and teeth, is limited to a restricted number of genes, including Jagged2, Jagged1,Notch1, Notch2, and Hes1. However, the cellular and molecular mechanisms involved in each development alteration as a result of function loss in each of these genes are not yet clear. It is therefore necessary to use animal models such as mice, chicken and zebrafish to analyze the expression of each NOTCH pathway gene in every stage of development and, through studies of loss and gain of gene function, to establish the function of each gene in early and late stages of craniofacial development.
On the other hand, in addition to further research on the cellular and molecular aspects of NOTCH pathway in craniofacial development, it is necessary to perform genetic studies on populations affected by craniofacial defects such as cleft palate and facial asymmetry, in order to study the presence of mutations in several NOTCH pathway genes that have been associated with these defects. Taking into account the existing gaps concerning the function of NOTCH pathway in craniofacial development, it is possible to raise the following questions in order to direct new research: what is the prevalence of genes Hes1 and Jagged2 mutations in cleft palate patients? Apart from Alagille patients, in what other patients with facial asymmetry can NOTCH pathway gene mutations be identified, besides Jagged1 and Notch2?
REFERENCES
1. Gross JB, Hanken J. Review of fate-mapping studies of osteogenic cranial neural crest in vertebrates. Deve Biol 2008; 317(2): 389-400. [ Links ]
2. Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol 2005; 284(1): 48-61. [ Links ]
3. Szabo-Rogers HL, Smithers LE, Yakob W, Liu KJ. New directions in craniofacial morphogenesis. Dev Biol 2010; 341(1): 84-94. [ Links ]
4. Chambers D, McGonnell IM. Neural crest: facing the facts of head development. Trends Genet 2002; 18(8): 381-384. [ Links ]
5. Le Douarin NM, Brito JM, Creuzet S. Role of the neural crest in face and brain development. Brain Rev 2007; 55(2): 237-247. [ Links ]
6. Helms JA, Cordero D, Tapadia MD. New insights into craniofacial morphogenesis. Development. 2005; 132(5): 851-861. [ Links ]
7. Cordero DR, Brugmann S, Chu Y, Bajpai R, Jame M, Helms JA. Cranial neural crest cells on the move: their roles in craniofacial development. Am J Med Genet 2011; 155A(2): 270-279. [ Links ]
8. Nie X, Luukko K, Kettunen P. BMP signalling in craniofacial development. Int J Dev Biol 2006; 50(6): 511- 521. [ Links ]
9. Paiva KB, Silva-Valenzuela Md, Massironi SM, Ko GM, Siqueira FM, Nunes FD. Differential Shh, Bmp and Wnt gene expressions during craniofacial development in mice. Acta histochem 2010; 112(5): 508-517. [ Links ]
10. Casey LM, Lan Y, Cho ES, Maltby KM, Gridley T, Jiang R. Jag2-Notch1 signaling regulates oral epithelial differentiation and palate development. Dev Dyn 2006; 235(7): 1830-1844. [ Links ]
11. Loomes KM, Stevens SA, OBrien ML, Gonzalez DM, Ryan MJ, Segalov M et al. Dll3 and Notch1 genetic interactions model axial segmental and craniofacial malformations of human birth defects. Dev Dyn 2007; 236(10): 2943-2951. [ Links ]
12. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science 1999; 284(5415): 770-776. [ Links ]
13. Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV et al. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev 1998; 12(7): 1046-1057. [ Links ]
14. Mitsiadis TA, Graf D, Luder H, Gridley T, Bluteau G. BMPs and FGFs target Notch signalling via Jagged2 to regulate tooth morphogenesis and cytodifferentiation. Development 2010; 137(18): 3025-3035. [ Links ]
15. Akimoto M, Kameda Y, Arai Y, Miura M, Nishimaki T, Takeda A et al. Hes1 is required for the development of craniofacial structures derived from ectomesenchymal neural crest cells. J Craniofac Surg 2010; 21(5): 1443- 1449. [ Links ]
16. Warthen DM, Moore EC, Kamath BM, Morrissette JJ, Sanchez-Lara PA, Piccoli DA et al. Jagged1 (JAG1) mutations in Alagille syndrome: increasing the mutation detection rate. Hum Mutat 2006; 27(5): 436-443. [ Links ]
17. Emerick KM, Rand EB, Goldmuntz E, Krantz ID, Spinner NB, Piccoli DA. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology 1999; 29(3): 822-829. [ Links ]
18. McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA et al. Notch2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet 2006; 79(1): 169- 173. [ Links ]
19. Penton AL, Leonard LD, Spinner NB. Notch signaling in human development and disease. Semin Cell Dev Biol 2012; 23(4): 450-457. [ Links ]
20. Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev 2007; 28(3): 339- 363. [ Links ]
21. Pan Y, Liu Z, Shen J, Kopan R. Notch1 and 2 cooperate in limb ectoderm to receive an early Jagged2 signal regulating interdigital apoptosis. Dev Biol 2005; 286(2): 472-482. [ Links ]
22. Fiuza UM, Arias AM. Cell and molecular biology of Notch. J Endocrinol 2007; 194(3): 459-474. [ Links ]
23. Gordon WR, Arnett KL, Blacklow SC. The molecular logic of Notch signaling a structural and biochemical perspective. J Cell Sci 2008; 121(Pt 19): 3109-3119. [ Links ]
24. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science 1999; 284(5415): 770-776. [ Links ]
25. Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol 2008; 3: 587-613. [ Links ]
26. Aster JC. Deregulated NOTCH signaling in acute T-cell lymphoblastic leukemia/lymphoma: new insights, questions, and opportunities. Int J Hematol 2005; 82(4): 295-301. [ Links ]
27. Gritli-Linde A. Molecular control of secondary palate development. Dev Biol 2007; 301(2): 309-326. [ Links ]
28. Dudas M, Li WY, Kim J, Yang A, Kaartinen V. Palatal fusion - where do the midline cells go? A review on cleft palate, a major human birth defect. Acta histochem 2007; 109(1): 1-14. [ Links ]
29. Din SU. Atypical tongue-tie due to congenital tonguepalate fusion. J Coll Physicians Surg Pak 2003; 13(8): 459- 460. [ Links ]
30. Humphrey T. Palatopharyngeal fusion in a human fetus and its relation to cleft palate formation. Ala J Med Sci 1970; 7(4): 398-426. [ Links ]
31. Richardson RJ, Dixon J, Jiang R, Dixon MJ. Integration of IRF6 and Jagged2 signalling is essential for controlling palatal adhesion and fusion competence. Hum Mol Genet 2009; 18(14): 2632-2642. [ Links ]
32. Mitsiadis TA, Lardelli M, Lendahl U, Thesleff I. Expression of Notch 1, 2 and 3 is regulated by epithelial-mesenchymal interactions and retinoic acid in the developing mouse tooth and associated with determination of ameloblast cell fate. J Cell Biol 1995; 130(2): 407-418. [ Links ]
33. Mitsiadis TA, Hirsinger E, Lendahl U, Goridis C. Deltanotch signaling in odontogenesis: correlation with cytodifferentiation and evidence for feedback regulation. Dev Biol 1998; 204(2): 420-431. [ Links ]
34. Mitsiadis TA, Henrique D, Thesleff I, Lendahl U. Mouse Serrate-1 (Jagged-1): expression in the developing tooth is regulated by epithelial-mesenchymal interactions and fibroblast growth factor-4. Development 1997; 124(8): 1473-1483. [ Links ]
35. Mitsiadis TA, Regaudiat L, Gridley T. Role of the Notch signalling pathway in tooth morphogenesis. Arch Oral Biol 2005; 50(2): 137-140. [ Links ]
36. Valsecchi C, Ghezzi C, Ballabio A, Rugarli EI. JAGGED2: a putative Notch ligand expressed in the apical ectodermal ridge and in sites of epithelial-mesenchymal interactions. Mech Dev 1997; 69(1-2): 203-207. [ Links ]
37. Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol 1999; 147(1): 105-120. [ Links ]
38. Mustonen T, Tummers M, Mikami T, Itoh N, Zhang N, Gridley T et al. Lunatic fringe, FGF, and BMP regulate the Notch pathway during epithelial morphogenesis of teeth. Deve Biol 2002; 248(2): 281-293. [ Links ]
39. Felszeghy S, Suomalainen M, Thesleff I. Notch signalling is required for the survival of epithelial stem cells in the continuously growing incisor. Differentiation 2010; 80(4- 5): 241-248. [ Links ]
40. Kamath BM, Loomes KM, Oakey RJ, Emerick KE, Conversano T, Spinner NB et al. Facial features in Alagille syndrome: specific or cholestasis facies? Am J Med Genet 2002; 112(2): 163-170. [ Links ]
41. Yuan ZR, Kohsaka T, Ikegaya T, Suzuki T, Okano S, Abe J et al. Mutational analysis of the Jagged 1 gene in Alagille syndrome families. Hum Mol Genet 1998; 7(9): 1363- 1369. [ Links ]
42. Kamath BM, Bauer RC, Loomes KM, Chao G, Gerfen J, Hutchinson A et al. NOTCH2 mutations in Alagille syndrome. J Med Genet 2012; 49(2): 138-144. [ Links ]
43. Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet 2012; 20(3): 251-257. [ Links ]
44. Kamath BM, Stolle C, Bason L, Colliton RP, Piccoli DA, Spinner NB et al. Craniosynostosis in Alagille syndrome. Am J Hum Genet 2002; 112(2): 176-180. [ Links ]
45. Piccoli DA, Spinner NB. Alagille syndrome and the Jagged1 gene. Semin Liver Dis 2001; 21(4): 525-534. [ Links ]
46. Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, Matthews RP et al. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development 2004; 131(22): 5753- 5766. [ Links ]
47. McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development 2002; 129(4): 1075- 1082. [ Links ]
48. Zuniga E, Stellabotte F, Crump JG. Jagged-Notch signaling ensures dorsal skeletal identity in the vertebrate face. Development 2010; 137(11): 1843-1852. [ Links ]
49. Humphreys R, Zheng W, Prince LS, Qu X, Brown C, Loomes K et al. Cranial neural crest ablation of Jagged1 recapitulates the craniofacial phenotype of Alagille syndrome patients. Hum Mol Genet 2012; 21(6): 1374- 1383. [ Links ]
50. Yen HY, Ting MC, Maxson RE. Jagged1 functions downstream of Twist1 in the specification of the coronal suture and the formation of a boundary between osteogenic and non-osteogenic cells. Dev Biol 2010; 347(2): 258-270. [ Links ]
51. Zanotti S, Canalis E. Notch signaling in skeletal health and disease. Eur J Endocrinol 2013; 168(6): R95-103. [ Links ]
52. Zanotti S, Canalis E. Notch regulation of bone development and remodeling and related skeletal disorders. Calcif Tissue Int 2012; 90(2): 69-75. [ Links ]
53. Isidor B, Lindenbaum P, Pichon O, Bézieau S, Dina C, Jacquemont S et al. Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nat Genet 2011; 43(4): 306-308. [ Links ]
54. Mucchielli ML, Mitsiadis TA. Correlation of asymmetric Notch2 expression and mouse incisor rotation. Mech Dev 2000; 91(1-2): 379-382. [ Links ]











 text in
text in