Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Odontología Universidad de Antioquia
Print version ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.26 no.2 Medellín Jan./June 2015
ORIGINAL ARTICLES DERIVED FROM RESEARCH
CONVENTIONAL DENTIN BONDING. DIFFICULTIES AND PROGRESS IN THE TECHNIQUE1
Ramos Sánchez Gisela2; Calvo Ramirez Norberto3; Fierro Medina Ricardo4
1 This work is part of the requirement for a professional degree; therefore,
it is not connected to any business relationship. Universidad Nacional
de Colombia. Research Project funded by Grupo de Investigación en
Materiales Dentales (GRIMAD) of Universidad Nacional de Colombia
2 MSc in Dentistry, Universidad Nacional de Colombia. E-mail: gisers@hotmail.com
3 Oral Rehabilitator Specialist. Professor, Universidad Nacional de
Colombia
4 PhD. Professor, Department of Chemistry. Laboratory of
Organometallic Compounds. Universidad Nacional de Colombia
SUBMITTED: OCTOBER 22/2013-ACCEPTED: JULY 22/2014
Ramos G, Calvo N, Fierro R. Conventional dentin bonding. Difficulties and progress in the technique. Rev Fac Odontol Univ Antioq 2015; 26(2): 468-486.
ABSTRACT
INTRODUCTION: studies on dentin bonding have reported that, contrary to the achieved stability on dental enamel, adhesive mechanisms
on dentine are still sensitive, unpredictable, and unstable. The objective of this study is to review the current literature on dentin bonding in order
to characterize conventional bonding, describing current modifications of the conventional protocol aimed at improving the adhesive performance
of dental materials.
METHODS: a literature review was conducted within 3 databases: ScienceDirect, Springer, and Medline, choosing the 52 most
relevant articles published between 2004 and 2013. The following key words were used as search criteria: dentin, dentin bonding, bond strength,
and acid etching.
RESULTS: the review of the selected articles provided a description of the conventional adhesion protocol showing the formation of
smear layer, the action of phosphoric acid, and the actual formation of adhesive interface, as well as the difficulties of the technique and possible
solutions suggested to date.
CONCLUSIONS: conventional dentin bonding is a precise and delicate procedure that shows disadvantages such as
the hydrolytic and proteolytic degradation of collagen matrix by enzymes released at the time of demineralization, which damages the adhesive
interface. Therefore, several substances have been suggested to be used as agents of collagen protection without altering adhesive strength, and
even improving it.
Key words: adhesives, composite resins, dentin coatings, dental cements, phosphoric acid.
INTRODUCTION
Modern restorative dentistry has shown rapid progress in dental adhesives technology in the last 50 years,1 achieving the original shape and color of natural teeth, preserving tooth structure through the so-called minimally-invasive dentistry. However, the clinical duration of composite resins is still very short due to incomplete hybridization at the adhesive interface, originating an area of exposed and unprotected collagen.2 Therefore, the technique of conventional dentin bonding is considered unstable since the tissue's heterogeneous composition does not enable ideal adhesive bonding, and inversely it may be affected by the hydrolytic degradation of hydrophilic monomers3 in the adhesive systems and by the action of the metalloproteinases that break down the exposed collagen fibers. This results in loss of retention of adhesive restorations, increased bacterial microleakage, secondary caries, and irreversible pulp alterations.4 Therefore, it is necessary to characterize and assess dentin, the conventional adhesion protocol, and current technical advances in order to use this knowledge as the basis for future research seeking the improvement of adhesive materials performance.
CHARACTERISTICS OF THE DENTINAL TISSUE
Dentine is composed of a calcium phosphate ore known as dahllite,5 which appears as small crystals of carbonate hydroxyapatite measuring 36 nm x 25 nm x 4 nm, and an organic phase whose main component is type l collagen in 90%, arranged in the form of a mesh. Its structure also includes small amounts of other types of collagen (IV, V, and VI) and other components such as phosphorylated and non-phosphorylated non-collagen proteins, proteoglycans, mucopolysaccharides, and lipids.2
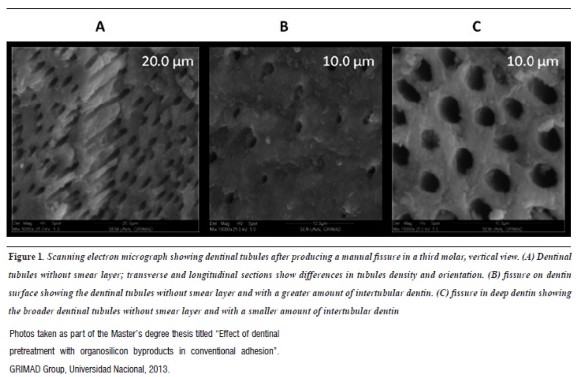
Ivancik et al6 and Shrivastava et al7describe how the structural characteristics of dentin, such as dentinal tubules, geometrically depend on the location within the tooth and the distance between pulp tissue and dentin. In general, the tubules are 1 to 2.5 µm in diameter with a density of 10,000 to 60,000 per mm-2 and each tubule is surrounded by peritubular dentine with a thickness of 0.5 to 1 µm; the region in between the tubules is known as intertubular dentin (figure 1) , mainly composed of a mesh of fibrillar collagen supported on apatite crystals.6, 8 Dentin is therefore a highly permeable tissue with tubules as well as micro-pores and micro-cracks that may originate in the enamel surface.
Dentine responds to mechanical and chemical stimuli from the environment thanks to its sensorial and mechanical properties, specifically elastic modulus. These anatomical characteristics determine the conditions of permeability and moisture, as well as physical properties like strength and elasticity.9, 10
INVOLVEMENT OF SMEAR LAYER IN THE ADHESIVE PROCESS
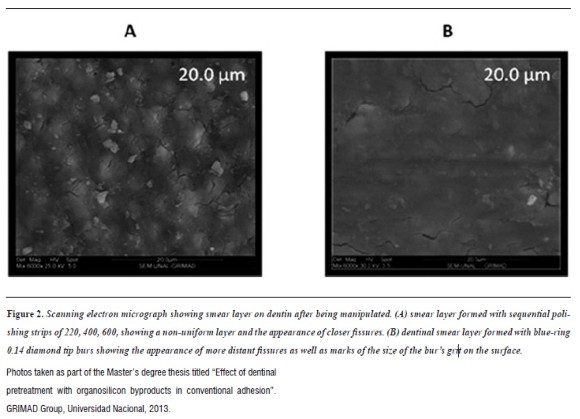
Whenever dentinal tissue is manipulated manually or with rotary instruments, a layer of detritus or waste, called smear layer, is formed on the surface.11 This layer is considered to be an obstacle in adhesive dentistry, since a suitable protocol of conventional adhesion is achieved by removing the smear layer from the surface with the use of phosphoric acid.
As indicated by Eldarrata et al,12 the smear layer is approximately 0.5 µm thick and it forms on teeth by organic components such as hydroxyapatite, saliva, blood, and bacteria. Smear layer is composed of two amorphous coatings: superficial layer and deep layer, the latter can extend up to 110 µm within the dentinal tubules and is called smear plug.12 Smear layer seals the adhesive interface and does not contribute to adhesive-dentin bonding.
Two groups of authors have reported that manipulation of dentinal tissue11, 13 can be done with polishing strips, burs, or polishing disks, producing varying thickness, roughness, density, and degree of adherence to smear layer to dentine according to instrumentation type.14Therefore, thickness of this layer varies between 0.5 and 2 µm.15 Thus, the smear layer formed by polishing strips leaves wider dentin tubules than those left by dental burs, considering that the different types of burs, in terms of grain size, provide different qualitative and quantitative characteristics (figure 2). Smear layer has been a source of controversy in the literature, with authors like Phasley et al2on the one hand, who promote the removal of this layer to facilitate impregnation of adhesives in dentinal tubules and demineralized collagen—a basic principle of conventional adhesion—2 and Van Meerbeeky et al16 on the other hand, who endorse the conservation of smear layer to reduce the number of clinical steps and the incidence of post-operative sensitivity.
ACTION OF PHOSPHORIC ACID ON DENTINAL TISSUE
In spite of the complexity of the organic and inorganic structure of dentin, it can be modified with the use of pre-conditioning acid agents17 that can generate varying porosities which may alter the physical and morphological characteristics of dentinal tubules. Dentinal pretreatment with phosphoric acid is designed to remove the dentinal smear layer and produce roughness on the surface through demineralization, which allows improving adhesion of polymeric resins to dental substrate.17, 18
Therefore, phosphoric acid has the ability of increasing inter and intra-tubular dentin permeability, as described by authors like Brajdiey et al18 and Shellisy et al,19 by dissolving the inorganic phase of dentin in a range of 3-7 µm. This permeability, contrary to the desired effect, is associated with postoperative hypersensitivity, generated when dentin is subjected to mechanical or thermal stimuli as a result of the widening of tubules after acid etching. This means that, besides seeking dentinal surface roughness to decrease the angle of contact of adhesive materials with the dentinal surface and thus obtaining greater moistening and adhesion,20 the acid-etching technique can also generate unwanted side effects.
Similarly, by increasing dentinal tubules size, phosphoric acid produces dentinal fluid leakage due to hydrostatic pressure, which can also weaken the interaction of chemical bond between monomers and dentin.21 It has recently been reported that before the dentine is demineralized with phosphoric acid, it contains 50% of mineral elements, 30% collagen, and 20% water.2 In demineralizing, 50% of the mineralized interface solubilizes and takes up 70% of new water content and 30% of collagen fibers anchored in the dentine's mineralized base.2 It would be ideal that this 70% were occupied by monomers polymerizing in situ to produce a biocompound strengthened with collagen fibers. However, in contradiction, the presence of residual solvents and the movement of fluid within the dentinal tubules inhibit the 70% water substitution by monomers to occur in an ideal manner.2
ADHESIVE STABILITY IN THE HYBRID LAYER
It is currently accepted that the base of adhesion to dentin is made of a structure called hybrid layer (figure 3), which is 3 to 6 µm thick. It is an intermediate zone made of collagen fibers and adhesive agent, located between the dentin and the restoration; it forms as a result of infiltration of the adhesive as a fluid between collagen fibers, since the mineral phase is already dissolved by phosphoric acid.2, 15 Based on numerous morphological research projects, some studies have shown that adhesive bonding depends on several factors, including moisture and depth of dentinal substrate, penetration of the adhesive agent through tubules, their intersection with collagen fibers exposed in the intertubular demineralized dentin, and the adhesive's components.22
Similarly, due to anatomical characteristics of dentinal tissue, the hybrid layer is different in superficial dentin and deep dentin. The first one is mainly composed of intertubular demineralized dentin, and to a lesser extent of the resin tags which are funnel-shaped and have more difficulty in penetrating through the narrowest dentinal tubules.23 Conversely, deep dentin contains less intertubular demineralized dentin, but its tubules are larger and more numerous and therefore the resin tags represent a significant fraction of the surface bonds near the pulp. This is why some authors claim that penetration and primer of the adhesive in conditioned dentin creates bonds with collagen, generating chemical and micromechanical retention and formation of tags,24 which contribute with 30% of the total strength of the adhesive bond.
Conventional adhesives include three-step adhesive systems, which require three bottles containing the demineralizing agent, the primer, and the bonding, and two-step adhesive systems, which include two containers, one with the demineralizing agent and one with the primer and the bonding in a single mixture.25 Conventional two-step adhesive systems are nowadays the most commonly used because they simplify the number of clinical steps, but evidence shows that eventually they experience adhesive strength alterations, probably due to the fact that a single bottle contains the hydrophilic components of the primer and the hydrophobic components of the adhesive, i.e., their composition can contain up to 50% of solvents, which increases the potential of absorbing water from the underlying dentin and the oral cavity, making the adhesive layer less stable.26 Furthermore, the greater the content of solvent in the adhesive solution before light curing, the lower the degree of conversion and therefore the mechanical properties of the adhesive are affected.23
Similarly, the authors state that, contrary to the expected uniformity, the adhesive's components are differently distributed in the exposed collagen. Hydrophilic monomers such as 2-hydroxyethyl methacrylate (HEMA) are limited to the lower half, near the dentin, being the most subjected to stress during the masticatory function, which can produce failures due to fatigue of the collagen fibers, and hydrophobic monomers such as bisphenol glycidylmethacrylate (BIS-GMA) are restricted to the upper half of the hybrid layer, close to the resin. The most significant problem of the adhesive interface is then that, even after light curing, the HEMA ester group is most vulnerable to hydrolytic dissociation, forming methacrylic acid and ethylene-glycol in the presence of basic- and acid-pH water.2 Also, once dentin is demineralized, diffusion of the adhesive monomers does not occur in the entire exposed collagen,27 resulting in an area in the lower part of the hybrid layer where collagen fibers are at risk of degradation and enzymatic hydrolysis.28
Authors such as Hashimoto et al29 and Carrilho at al30 have reported that this area contains free dentinal matrix enzymes called metalloproteinases, endogenous proteases that are present in dentin from developmental stages and that remain exposed once dentin is demineralized, which suggests that prevention of enzymatic degradation of collagen is a potential strategy to improve adhesive bonding.29, 30 There are several types of metalloproteinases (MMP), collagenases (MMP-8), gelatinases (MMP-2), and enamelysines (MMP-20), which are responsible for collagen loss and therefore for the continuity of the hybrid layer, which causes decreased retention of polymeric material to dentin.31 Hence collagen fibrils degrade in the hybrid layer and, as a direct result, the adhesive-dentin interface disappears, which suggests that, due to stability of adhesion to enamel, sealing the resin in the restoration periphery would be a great contribution to adhesion durability, which is not sufficient to support the flexion and compression forces generated by cyclic tension during the masticatory function.
For this reason, recent studies in the field of dentinal bonding seek to find ways to inhibit the effect of metalloproteins. Different substances have been tested, including chlorhexidine, considered by the authors as a powerful inhibitor of metalloproteinases at very low concentrations.32, 33 A concentration of 0.2% digluconate of chlorhexidine is able to inhibit 99% of the collagenolitic activity of metalloproteinases in vitro. This process is under study, since the mechanism of the inhibitory process is still unknown.34
This way, not only chlorhexidine has been used as a dentinal pretreatment to improve performance of the adhesive interface, but also sodium hypochlorite (NaOCl) has been used as a denaturation and deproteinization agent capable of eliminating collagen, in agreement with the concept of collagen mesh elimination as a way to increase adhesive systems stability.35 It has been suggested to create a dentin layer with similar characteristics to those of etched enamel, i.e., a greater presence of hydroxyapatite crystals with high surface energy.36 The results are due to the fact that deproteinized dentin has higher hardness, greater humidifying capacity, and greater permeability than demineralized dentin; however, despite the apparent advantages, there is not enough information on how hypochlorite could affect residual collagen fibers, as well as on the effects of pulp biocompatibility and the effects of interaction with adhesive resins.
On the other hand, according to Zhang et al37 and Pascon et al,38 hypochlorite creates submicron porosity within the mineral phase and increases the size of tubules due to loss of peritubular and intertubular dentin, producing extension and coalescence of dentinal tubules if used as irrigant in root canals. Hypochlorite eliminates both organic matter and ions of magnesium and carbonate, which alter the mechanical properties of dentin and the sealing capacity of dental materials. Similarly, Kaya et al39 and Prasansuttiporn et al40 report that the alteration created by hypochlorite in dentin is proportional to its concentration. Attrition caused by a concentration of 1.3% NaOCl is lower than that caused by a concentration of 5.25% NaOCl.39 Even if hypochlorite is mixed with solutions such as ethylenediaminetetraacetic acid (EDTA), dentin attrition grows proportionally to the concentration and exposure time, progressively decreasing adhesion strength and mechanical properties.
It has been recently shown that sodium hypochlorite (NaOCl) reduces the bonding strength of resin compounds and dentin, since debris and byproducts generated by hypochlorite have a negative effect on the polymerization of adhesive systems.40 Therefore, researchers have attempted to use antioxidants such as ascorbic acid—solutions with antioxidant capacities and with the power to inhibit MMPs, which would improve the adhesive capacity of treated dentin.
Within the substances used as dentinal pretreatment, ethylenediaminetetraacetic acid (EDTA) is a polyvalent ligand that acts as a chelating agent trapping ion Zn+2, needed by metalloproteinases to keep their hydrolase catalytic activity; similarly, the ion Ca+2 allows these enzymes to maintain their tertiary structure.2, 40 Dentinal conditioning with EDTA 0.5 M for 1 to 2 minutes in ethanol creates a thinner hybrid layer, where hydrophobic monomers would infiltrate entirely without leaving adhesive-free spaces, as happens when using phosphoric acid, which would improve adhesion and microtensil strength.41 It can also inactivate metalloproteinases as well as other chelators, such as 1.10-Phenanthroline and etilendiaminotetraphosphoric acid, which could result in a better long-term conservation of the hybrid layer in both healthy dentin and cariesaffected dentin.
Substances proposed for metalloproteinase inhibition include the so-called protein crosslinking agents, so that endogenous metalloproteinases are linked to their peptide chains immediately after acid-etching, losing molecular mobility which is essential for enzyme activity.2Guentsch et al42 and Ishihata et al43 highlight glutaraldehyde as a very effective crosslinking agent whose negative effect is cytotoxicity for dental pulp and potential carcinogenicity.42 Glutaraldehyde in aqueous solution of 5 and 35% 2-hydroxyethyl methacrylate (HEMA) has been used in the management of dental hypersensitivity,43 showing coagulation of plasma proteins and therefore tubular blocking after topical application in hypersensitive dentin.
The authors have described new crosslinking agents that are less toxic for tissues: the socalled proanthocyanidins and carbodiimides.44 Proanthocyanidins (PA) are antioxidant vegetal flavonoid compounds usually found in a wide variety of fruits, vegetables, flowers, nuts, seeds, and barks. Their great advantage is that they stimulate healthy tissues and have been proven to increase resin-dentine bond strength.45 The disadvantage is that their action time lasts 10 to 30 minutes, and that would not clinically applicable.
The action of these substances is currently being tested in terms of time, achieving shorter intervals by using carbodiimides. Proanthocyanidins can be extracted from grape seeds. This extract promotes bone formation in rats' mandibular condyles,increases rigidity of demineralized dentin, stops caries progression in artificial roots, and prevents the production of metalloproteinases.46 Its effects are similar to those of the extracts of bilberries and elm trees bark. The studies on proanthocyanidins are very recent but they have achieved inhibition of collagen degradation in the adhesive interface and improved resistance to traction and rigidity.
Recent studies have reported pretreatment of demineralized dentin with proanthocyanidins for 1 hour before initiating the adhesive protocol, yielding excellent results even in caries-affected dentine.47 Due to the required long periods, some studies have suggested to apply proanthocyanidins directly on the adhesive systems, showing good results in decreasing nanofiltration without affecting bond strength.48, 49 This brings about a new concept for research: dentin biomodification, which seeks to improve the biomechanical and biochemical properties of the organic matrix through covalent and non-covalent bonds within the collagen, which are able to form intra- and inter-molecular crosslinks thanks to lysine hydroxylation and the rate of molecular rotation, which will finally provide collagen with greater stability.45
Tezvergily et al50 and Kimay et al51 highlight the use of polyvinyl phosphoric acid (PVPA), a substance with anti-collagenolytic activity which electrostatically bonds to dentin collagen and can be trapped in matrices by crosslink agents through 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide in 1 to 5 minutes, minimizing collagen loss due to ionic competition. PVPA has also been used in biomimetic mineralization models in such a way that coupled with collagen fibers it can guide the distribution of apatite crystals because it mimics the negative charges of phosphoproteins such as bone sialoprotein and phosphoserine phosphoporine.
Riboflavin has been recently introduced as a type I collagen cross-linking agent able to increase the stability of collagen fibers, increase mechanical properties, and reduce enzymatic degradation, providing demineralized dentin with greater efficiency.52 Riboflavin has shown increased biomechanical resistance of human cornea in the treatment of keratoconus; this is why the action of riboflavin has been proven to be a crosslinking agent in demineralized human dentin. Riboflavin produces free radicals when it is photo-activated with wavelengths of 270, 366 and 445 nm,53 in such a way that reactive oxygen species are released and light is absorbed, forming crossed covalent bonds between adjacent collagen molecules. This way, the effect of activation by riboflavin UVA rays (long wave ultraviolet radiation) increases immediate bond strength to dentin, stabilizing the adhesive interface and inhibiting metalloproteinases.47
In this way, current studies seek to link monomers of adhesive resins with substances capable of forming crossed anti-metalloproteinase bonds that directly combine with collagen dentin to form a hybrid layer with greater durability potential.50
DISCUSSION
This literature review shows that the current conventional protocol used in the process of adhesion to dentinal tissue has flaws related to the duration and stability of the bond, which is still sensitive and unpredictable as published by Pashley in 2011.2, 50 Experience has shown that resin restorations need to be replaced every 5.7 years on average due to adhesive interface loss, which leads to fissures and secondary caries because of bacterial microleakage.4 This suggests the need to search for methods to increase the durability of resinous restorations.
In addition, the authors demonstrate the difficulties related to the typical characteristics of dentinal substrate due to its heterogeneous constitution and its physiologically dynamic behavior, which may vary under normal and pathological clinical situations.2
The dentinal fluid and the formation of smear layer on the surface of the dentinal substrate—which can also reach intratubular levels once instrumentation is performed— are determinant variables at the time of conducting an adhesion protocol. In conventional adhesion, such substrate is prepared with 37% phosphoric acid, generating demineralization which in turn enlarges the dentinal tubules and exposes the dentin's collagen matrix, creating a surface ready and available for humidification of the adhesive.17, 18 The use of conditioner acid and the subsequent exposure of collagen mesh once calcium is removed are considered critical moments in adherence to the dentinal substrate, since most clinical errors and technical problems happen during these process.
The literature suggests that demineralization produced by phosphoric acid is capable of altering the dentin's phosphate and calcium ores more deeply than the adhesive system is able to penetrate, forming an area of exposed collagen vulnerable to mechanical damage and hydrolytic degradation.29, 30 Consequently, one of the bases of current research focuses on inhibiting the activity of zinc-dependent endopeptidases, also called metalloproteinases, with the help of substances used as dentinal pretreatments before applying the adhesive agent during the conventional protocol.
Several substances have been tested to date, including hypochlorite sodium, EDTA, glutaraldehyde, and substances that have not yielded good results as dentinal pretreatments due to their side or collateral biochemical effects, which eventually decrease biocompatibility.37, 41, 43 On the other hand, some other authors state that substances such as chlorhexidine and antioxidant substances more compatible with pulp tissue such as proanthocyanidins and carbodiimides, as well as vegetable flavonoid tissues of natural origin obtained from vegetables and fruits are able to inhibit collagen degradation and improve tensile strength and rigidity.44, 47
Similarly, researchers recognize the effect of polyvinyl phosphoric acid in biomimetic mineralization models, as well as the properties of riboflavin, which is capable of increasing the strength of immediate bond to dentin.50 So far, the results of using these substances have been positive and satisfactory, but further biocompatibility studies are needed in order to clarify the mechanism of action of these types of dentinal pretreatment on pulp tissue, as well as mechanical studies on thermo-cycling or hardening of the adhesive interface that show the behavior of these substances in the long term.
Similarly, the stresses to which the dentin is subjected during mastication, swallowing, and other parafunctional habits also challenge the durability of the adhesive interface, since a mechanically damaged collagen is more susceptible to proteolysis. Therefore, its durability should also be evaluated through mechanical loading in order to establish parallels between biomechanical and biochemical changes.
CONCLUSIONS
Dentin is a porous biological compound formed by apatite supported on a collagen matrix whose structural characteristics provide it with properties determined by the anatomical condition of dentinal tubules, pulp fluid, and tissue depth. Therefore, the protocol of conventional adhesion to dentin is a precise and sensitive procedure which has shown flaws related to hydrolytic and proteolytic degradation of collagen by enzymes from the dentin, which are released at the time of demineralization and damage the adhesive interface. Therefore, current research seeks substances that can be used as collagen protection agents, achieving greater durability of adhesive restorations in dentin without altering and even improving mechanical properties—a goal that has yielded promising results so far.
ACKNOWLEDGMENTS
This study was sponsored by the School of Dentistry of Universidad Nacional de Colombia at Bogotá. It was conducted as part of the Master's Degree in Dentistry with a focus on Dental Materials, with the direction of Dr. Ricardo Fierro Medina and Dr. Norberto Calvo Ramírez, and the support of Dr. Clementina Infante Contreras, Coordinator of the Master's Program.
CONFLICT OF INTEREST
None of the authors has declared conflicts of interest.
REFERENCES
1. Van Meerbeek B, Peumans M, Poitevin A, Mine A, Van Ende A, Neves A et al. Review relationship between bondstrength tests and clinical outcomes. Dent Mater 2010; 26: e100-121. [ Links ]
2. Pashley DH, Tayb FR, Breschic L, Tjäderhanee L, Carvalhof RM, Carrilhog M et al. State of the art etch-andrinse adhesives. Dent Mater 2011; 27: 1-16. [ Links ]
3. Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007; 28: 3757-3785. [ Links ]
4. Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol 2007; 52: 121-127. [ Links ]
5. Zaslansky P, Zabler S, Fratzl P. 3D variations in human crown dentin tubule orientation: A phase-contrast microtomography study. Dent Mater 2010; 26: e1-10. [ Links ]
6. Ivancik J, Majd H, Bajaj D, Romberg E, Arola D. Contributions of aging to the fatigue crack growth resistance of human dentin. Acta Biomater 2012; 8: 2737- 2746. [ Links ]
7. Shrivastava S, Aifantis Katerina E. Effects of cola drinks on the morphology and elastic modulus of dentin. Mater Lett 2011; 65: 2254-2256. [ Links ]
8. Elbaum R, Tal E, Perets AI, Oron D, Ziskind D, Silberberg Y et al. Dentin micro-architecture using harmonic generation microscopy. J Dent 2007; 35: 150-155. [ Links ]
9. Arola D, Reprogel RK. Tubule orientation and the fatigue strength of human dentin. Biomaterials 2006; 27: 2131- 2140. [ Links ]
10. Zaslansky P. Dentin. En: Fratzl P. Collagen: structure and mechanics. New York: Springer; 2008. [ Links ]
11. Sattabanasuk V, Vachiramon V, Qian F, Armstrong SR. Resin-dentin bond strength as related to different surface preparation methods. J Dent 2007; 35: 467-475. [ Links ]
12. Eldarrata AH, High AS, Kale GM. In vitro analysis of 'smear layer' on human dentine using ac-impedance spectroscopy. J Dent 2004; 32: 547-554. [ Links ]
13. Nakajima M, Kunawarote S, Prasansuttiporn T, Tagami J. Bonding to caries-affected dentin. Jpn Dent Sci Rev 2011; 47: 102-114. [ Links ]
14. Oliveiraa SS, Pugach MK, Hiltonb JF, Watanabe LG, Marshall SJ, Marshall GW Jr. The in?uence of the dentin smear layer on adhesion: a self-etching primer vs. a totaletch system. Dent Mater 2003; 19: 758-767. [ Links ]
15. Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O et al. Adhesive/Dentin Interface: The Weak Link in the composite restoration. Ann Biomed Eng 2010; 38: 1989- 2003. [ Links ]
16. Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dent Mater 2011; 27: 17-28. [ Links ]
17. Farge P, Alderete L, Ramos SM. Dentin wetting by three adhesive systems: Influence of etching time, temperature and relative humidity. J Dent 2010; 38: 698-706. [ Links ]
18. Brajdic D, Krznaric O M, Azinovic Z, Macan D, Baranovic M. Influence of different etching times on dentin surface morphology. Coll Antropol 2008; 32: 893-900. [ Links ]
19. Shellis RP, Curtis AR. A minimally destructive technique for removing the smear layer from dentine surfaces. J Dent 2010; 38: 941-944. [ Links ]
20. Ramos SM, Alderete L, Farge P. Dentinal tubules driven wetting of dentin: Cassie-Baxter modelling. Eur Phys J E 2009; 30: 187-195. [ Links ]
21. Miyazaki M, Tsubota K, Takamizawa T, Kurokawa H, Rikuta A, Ando S. Factors affecting the in vitro performance of dentin-bonding systems. Jpn Dent Sci Rev 2012; 48: 53-60. [ Links ]
22. Wang Y, Yao X. Morphological/chemical imaging of demineralized dentin layer in its natural, wet state. Dent Mater 2010; 26: 433-442. [ Links ]
23. Fawzy AS. Variations in collagen fibrils network structure and surface dehydration of acid demineralized intertubular dentin: effect of dentin depth and air-exposure time. Dent Mater 2010; 26: 35-43. [ Links ]
24. Langer A, Llie N. Dentin infiltration ability of different classes of adhesive systems. Clin Oral Invest 2012; 17: 205-216. [ Links ]
25. Reis A, de Carvalho Cardoso P, Vieira LC, Baratieri LN, Grand RH, Loguercio AD. Effect of prolonged application times on the durability of resin-dentin bonds. Dent Mater 2008; 24: 639-644. [ Links ]
26. Proença JP, Polido M, Osorio E, Erhardt MC, Aguilera FS, García-Godoy F et al. Dentin regional bond strength of self-etch and total-etch adhesive systems. Dent Mater 2007; 23: 1542-1548. [ Links ]
27. Erhardt MC, Osorio R, Toledano M. Dentin treatment with MMPs inhibitors does not alter bond strengths to cariesaffected dentin. J Dent 2008; 36: 1068-1073. [ Links ]
28. Fang M, Liu R, Xiao Y, Li F, Wang D, Hou R et al. Biomodification to dentin by a natural crosslinker improved the resin-dentin bonds. J Dent 2012; 40: 458- 466. [ Links ]
29. Hashimoto M, Nagano F, Endo K, Ohno H. A review: biodegradation of resin-dentin bonds. Jpn Dent Sci Rev 2011; 47: 5-12. [ Links ]
30. Carrilho MR, Carvalho RM, Sousae EN, Nicolau J, Breschi L, Mazzoni A et al. Substantivity of chlorhexidine to human dentin. Dent Mater 2010; 26: 779-785. [ Links ]
31. Wang DY, Zhang L, Fan J, Li F, Ma KQ, Wang P et al. Matrix metalloproteinases in human sclerotic dentine of attrited molars. Arch Oral Biol 2012; 57: 1307-1312. [ Links ]
32. Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L et al. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater 2010; 26: 771-778. [ Links ]
33. Perdigão J. Dentin bonding -variables related to the clinical situation and the substrate treatment. Dent Mater 2010; 26: e24-37. [ Links ]
34. Zhou J, Tan J, Chen L, Li D, Tan Y. The incorporation of chlorhexidine in a two-step self-etching adhesive preserves dentin bond in vitro. J Dent 2009; 37: 807-812. [ Links ]
35. Hegde M, Bhide S. Nanoleakage phenomenon on deproteinized human dentin -an in vitro study. Indian J Dent 2012; 3(1): 5-9. [ Links ]
36. Correr GM, Alonso RC, Grando MF, Borges AF, Puppin- Rontani RM. Effect of sodium hypochlorite on primary dentin -A scanning electron microscopy (SEM) evaluation. J Dent 2006; 34: 454-459. [ Links ]
37. Zhang K, Tay FR, Kim YK, Mitchell JK, Kim JR, Carrilho M et al. The effect of initial irrigation with two different sodium hypochlorite concentrations on the erosion of instrumented radicular dentin. Dent Mater 2010; 26: 514-523. [ Links ]
38. Pascon FM, Kantovitz KR, Sacramento PA, Nobre-dos-Santos M, Puppin-Rontani RM. Effect of sodium hypochlorite on dentine mechanical properties. A review. J Dent 2009; 37: 903-908. [ Links ]
39. Kaya S, Yigit-Özer S, Adigüzel Ö. Evaluation of radicular dentin erosion and smear layer removal capacity of selfadjusting file using different concentrations of sodium hypochlorite as an initial irrigant. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 112(4): 524-530. [ Links ]
40. Prasansuttiporn T, Nakajima M, Kunawarote S, Foxton RM, Tagami J. Effect of reducing agents on bond strength to NaOCl-treated dentin. Dent Mater 2011; 27: 229-234. [ Links ]
41. Sauro S, Toledano M, Aguilera FS, Mannocci F, Pashley DH, Tay FR et al. Resin-dentin bonds to EDTAtreated vs. acid-etched dentin using ethanol wet-bonding. Dent Mater 2010; 26: 368-379. [ Links ]
42. Guentsch A, Seidler K, Nietzsche S, Hefti AF, Preshaw PM, Watts DC et al. Biomimetic mineralization: Long-term observations in patients with dentin sensitivity. Dent Mater 2012; 28: 457-464. [ Links ]
43. Ishihata H, Kanehira M, Finger Werner J, Shimauchi H, Komatsu M. Effects of applying glutaraldehyde-containing desensitizer formulations on reducing dentin permeability. J Dent Sci 2012; 7: 105-110. [ Links ]
44. Green B, Yao X, Ganguly A, Xu C, Dusevich V, Walker MP et al. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J Dent 2010; 38: 908-915. [ Links ]
45. Xie Q, Bedran-Russo AK, Wu CD. In vitro remineralization effects of grape seed extract on artificial root caries. J Dent 2008; 36: 900-906. [ Links ]
46. Bedran-Russo AK, Castellan CS, Shinohara MS, Hassan L, Antunes A. Characterization of biomodi?ed dentin matrices for potential preventive and reparative therapies. ActaBiomater 2011; 7: 1735-1741. [ Links ]
47. Epasinghe DJ, Yiu CK, Burrow MF, Tay FR, King NM. Effect of proanthocyanidin incorporation into dental adhesive resin on resin-dentine bond strength. J Dent 2012; 40: 173-180. [ Links ]
48. Liu Y, Wang Y. Effect of proanthocyanidins and photoinitiators on photo-polymerization of a dental adhesive. J Dent 2013; 41: 71-79. [ Links ]
49. Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater 2010; 26: 968-973. [ Links ]
50. Tezvergil-Mutluay A, Agee KA, Hoshika T, Tay FR, Pashley DH. The inhibitory effect of polyvinylphosphonic acid on functional matrix metalloproteinase activities in human demineralized dentin. Acta Biomater 2010; 6: 4136-4142. [ Links ]
51. Kim YK, Gu LS, Bryan TE, Kim JR, Chen L, Liu Y et al. Mineralisation of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium, phosphate and hydroxyl ions. Biomaterials 2010; 31: 6618-6627. [ Links ]
52. Fawzy AS, Nitisusanta LI, Iqbal K, Daood U, Beng LT, Neo J. Chitosan/Riboflavin-modified demineralized dentin as a potential substrate for bonding. J Mech Behav Biomed Mater 2013; 17: 278-289. [ Links ]
53. Fawzya AS, Nitisusanta LI, Iqbal K, Daood U, Neo J. Riboflavin as a dentin crosslinking agent: Ultraviolet A versus blue light. Dent Mater 2012; 28: 1284-1291. [ Links ]











 text in
text in