INTRODUCTION
With advancing technology, new approaches came into question in the field of animal nutrition. Use of wireless sensors is one of these new approaches. Recently, some studies are being conducted with the aim of determining the possibilities of using wireless rumen sensors (WRS=Bolus).
By using WRSs, some rumen parameters (pH, temperature and pressure) can be recorded continuously and with this way some data related to rumen fermentation can be obtained instantly. So, some nutrition related disorders can be determined in time 1-4. Furthermore, use of WRSs makes possible to add feeds which improve rumen health into ration. It is also thought that some remarkable achievements can be attained in prevention of subacut rumen acidosis (SARA) in ruminants 3,5-7. Besides, some valuable information related to bloat, animal welfare, mastitis, calving time, drinking behaviour, satiety and rumen-intestine movements can be obtained by using WSRs.
The lack of adequate studies on use of WRSs in ruminant nutrition decreases the reliability of obtained data. The reliable results obtained in limited studies 3,6,8,9, but not in other studies 6. Use of WSRs in animal nutrition experiments and on-farm studies is not common.
In the present study, it was aimed to determine the possibilities of using WRSs in integration with Daisy Incubator with the aim of determining the effects of straws pelleted with different additives on rumen fermentation. Furthermore, the hypothesis of the study was that while molasses and sepiolite addition to straws decreases rumen pH, guar meal addition increases rumen pH.
MATERIALS AND METHODS
Wireless rumen sensors. In the study, 5 WRSs (e-Cow) (Weight: 154.4 g; Length: 13.0 cm; Width: 2.71 cm) which were produced for use in scientific studies (cannulated animals), also, a receiver and a mobil vehicle, in which required software was recorded, were purchased from U.K. The battery life of boluses and optimum working temperatures of boluses were 90-150 days and 34-40°C, respectively. The boluses have a data storage capacity for 28 days. Each bolus was labeled with a different label (ID).
Rumen fluid collection. Rumen fluid collected from Simmental- breed cow (18 months age) with a developed rumen, slaughtered at a private salaughter house with a developed rumen was filtered through two layers of cheesecloth and then was brought to the laboratory in thermos (38-40°C). The pH value of rumen fluid was determined using digital pH meter (Hanna Instruments 1332) with 3 replications. The average rumen pH was determined as 6.15 (6.08-6.51). Ethics approval is not required in this study because, rumen fluid was collected from slaughterhouse.
Preparation of straw pellets and experimental design. Wheat and soybean straws were used as feed material in the study. Eight experimental groups (2 sepiolite applications (+ and -) and 4 different feed groups (control, guar meal, molasses and guar meal+molasses)) were formed for each straw. The application rates for sepiolite, molasses, guar meal and guar meal+molasses were 2, 7, 10 and 17%, respectively. These additives were mixed with straw and then 6 mm-sized pellets were prepared.
Chemical analyses of straw pellets. The samples were milled to pass through a 1 mm sieve before chemical analyses. The ground samples were analyzed for Kjeldahl nitrojen (N) and crude protein (CP) was calculated by multiplying N by 6.25. Dry matter (DM) and ash were determined according to AOAC 10. The neutral detergent fiber (NDF), acid detergent fiber (ADF), acid detergent lignin (ADL) and crude fiber (CF) analysis were done according to the method of Van Soest et al 11 using ANKOM 2000 semi-automated fiber analyzer (Ankom Technology). Ether extract (EE) was determined according to AOCS 12 Am-5-04 procedures by using ANKOM analyzer. The contents of nitrogen free extract (NFE) of sample straws were determined by calculation.
Determination of changes in vitro rumen pH and temperatures. The study was conducted between December 30, 2015 and January, 1 2016. Two liters of incubation fluid (1600 ml buffer solution+400 ml rumen fluid) were added to each of 4 jars in Daisy incubator. The bags filled with samples were incubated in the jars with purging CO2 for 48 hours at 39°C. The pH and temperature values for each treatment in wheat and soybean straws were determined by using WRSs inserted in each jar for 48 hours. The boluses were adjusted for one measurement per 15 minutes as reported by Hanusovsky et al 13. Prior to use, each bolus was calibrated for pH 4 and pH 7 via mobil vehicle, which contains software, and computer and then data flow was monitored at least for 20 minutes. The pH and temperature measurements of boluses were tested by using a digital pH meter (Hanna Instruments 1332). The boluses were stored in water bath (38°C) until use. The boluses were inserted in jars of Daisy incubator at the same time. Data were transferred from boluses to mobil vehicle for 6 hour intervals.
Statistical Analyses. Data related to in vitro rumen pH and temperatures were subjected to one way analysis of variance and Duncan test was used for the mean comparisons.
RESULTS
Chemical compositions of straw pellets. The nutrient contents and cell wall contents of the straw pellets are given in Table 1. Soybean straw had higher CF and CP contents compared to wheat straw (p<0.001). Furthermore, it is seen that use of additives lead to changes in nutrient composition of straw pellets. Thus, CP contents of pelleted straws increased due to use of guar meal. Soybean straws are shown to have higher ADL contents than wheat straws.
Table 1 Nutrient contents of feeds used in the experiment,%
| Samples | DM | Ash | CP | EE | CF | NFE | NDF | ADF | ADL |
|---|---|---|---|---|---|---|---|---|---|
| Wheat Cont- | 90.46 ±0.06e | 7.57 ±0.28de | 4.19 ±0.00e | 0.56 ±0.09 | 38.51 ±0.66de | 39.63 ±0.87a | 69.36 ±0.41b | 42.42 ±0.41def | 4.77 ±0.18e |
| Wheat Cont+ | 91.44 ±0.01c | 7.47 ±0.31de | 3.54 ±0.06e | 0.67 ±0.35 | 38.91 ±0.93cde | 40.85 ±1.36a | 72.49 ±0.72a | 43.48 ±0.46cde | 5.48 ±0.60e |
| Wheat M- | 88.90 ±0.05g | 8.19 ±0.22cd | 6.16 ±0.05d | 1.25 ±0.31 | 40.47 ±0.91b-e | 32.84 ±1.05cd | 68.05 ±0.51bc | 42.16 ±0.22def | 6.20 ±0.28e |
| Wheat M+ | 87.84 ±0.07i | 9.56 ±0.28b | 4.46 ±1.62e | 0.89 ±0.33 | 38.19 ±2.88de | 34.75 ±1.11bc | 66.13 ±0.56cd | 40.46 ±0.42f | 5.47 ±0.54e |
| Wheat G- | 91.84 ±0.03b | 6.92 ±0.15ef | 6.26 ±0.11d | 0.79 ±0.22 | 39.32 ±1.72b-e | 38.54 ±1.72ab | 69.48 ±0.44b | 42.01 ±0.69ef | 4.90 ±0.40e |
| Wheat G+ | 92.35 ±0.01a | 8.65 ±0.27c | 6.63 ±0.10d | 0.33 ±0.14 | 38.06 ±2.05de | 38.68 ±1.76ab | 69.16 ±0.41b | 42.03 ±0.14ef | 4.96 ±0.42e |
| Wheat GM- | 88.50 ±0.10h | 7.55 ±0.29de | 10.44 ±0.19a | 0.78 ±0.26 | 36.30 ±1.67e | 33.43 ±1.83cd | 65.44 ±0.28d | 40.44 ±0.23f | 5.16 ±0.07e |
| Wheat GM+ | 87.65 ±0.19i | 8.59 ±0.41c | 8.73 ±0.15bc | 0.62 ±0.12 | 38.04 ±2.43de | 31.67 ±2.63cde | 64.76 ±0.63d | 39.76 ±0.31f | 4.82 ±0.33e |
| Soybean Cont- | 88.76 ±0.12g | 7.57 ±0.30de | 7.46 ±0.17cd | 0.58 ±0.05 | 43.91 ±1.38abc | 29.24 ±1.45def | 66.59 ±0.44cd | 52.22 ±0.80a | 14.27 ±0.45a |
| Soybean Cont+ | 90.91 ±0.05d | 7.61 ±0.46de | 4.17 ±0.01e | 0.77 ±0.14 | 47.47 ±1.23a | 30.88 ±0.73cde | 65.90 ±0.03d | 52.15 ±0.40a | 11.55 ±0.18cd |
| Soybean M- | 84.68 ±0.12l | 10.04 ±0.20b | 6.95 ±0.03d | 0.51 ±0.43 | 42.69 ±1.28a-d | 24.50 ±0.54fg | 59.40 ±1.02e | 45.65 ±1.23bc | 13.44 ±0.12ab |
| Soybean M+ | 85.84 ±0.09k | 12.65 ±0.29a | 6.91 ±0.21d | 0.47 ±0.11 | 38.70 ±1.34cde | 27.13 ±1.25ef | 56.87 ±0.37f | 45.97 ±2.06bc | 11.93 ±1.14bc |
| Soybean G- | 90.20 ±0.12f | 13.34 ±0.19a | 10.08 ±0.44ab | 0.71 ±0.32 | 35.56 ±0.82e | 30.51 ±1.01cde | 52.80 ±0.59g | 40.86 ±0.88ef | 10.93 ±0.34cd |
| Soybean G+ | 90.13 ±0.03f | 12.94 ±0.22a | 9.48 ±0.07ab | 0.24 ±0.04 | 39.62 ±1.53b-e | 27.85 ±1.72ef | 56.35 ±1.37f | 42.30 ±0.85def | 10.27 ±0.14d |
| Soybean GM- | 84.01 ±0.09m | 6.38 ±0.38f | 10.69 ±0.64a | 0.69 ±0.37 | 44.2 ±1.09ab | 22.00 ±1.64g | 60.37 ±0.85e | 47.27 ±0.49b | 12.34 ±0.47bc |
| Soybean GM+ | 86.87 ±0.06j | 12.68 ±0.19a | 9.42 ±0.03ab | 0.62 ±0.28 | 42.6 ±2.00a-d | 21.52 ±2.14g | 59.12 ±0.73e | 44.77 ±1.10bcd | 12.10 ±0.77bc |
| Significant | <0.001 | <0.001 | <0.001 | 0.622 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| DM: Dry matter, CP: Crude protein, EE:Ether extract, CF: Crude fiber, NFE: Nitrogen free extracts, NDF: Neutral detergent fiber, ADF: Acid detergent fiber, ADL: Acid detergent lignin; Wheat Cont-: wheat control, Wheat Cont+: wheat control with sepiolite, Wheat M-:wheat molasses, Wheat M+: wheat molasses with sepiolite, Wheat G-: wheat guar meal, Wheat G+: wheat guar meal with sepiolite, Wheat GM-: wheat guar meal+molasses, Wheat GM+: wheat guar meal+molasses with sepiolite, Soybean Cont-: soybean control, Soybean Cont+: soybean control with sepiolite, Soybean M-:soybean molasses, Soybean M+: soybean molasses with sepiolite, Soybean G-: soybean guar meal, Soybean G+: soybean guar meal with sepiolite, Soybean GM-: soybean guar meal+molasses, Soybean GM+: soybean guar meal+molasses with sepiolite |
Determination of changes in vitro pH and temperature using wireless rumen sensors. The pH and temperature measurements of boluses were tested (Table 2) by using a digital pH meter. The pH and temperature values measured by using boluses were found similar to those measured by using digital pH meter. However, one (5 number bolus) of the boluses was not used in in vitro study due to calibration problems.
Table 2 Test results of wireless rumen sensors*
| Measurement | Bolus 1 | Bolus 2 | Bolus 3 | Bolus 4 | Bolus 5 | pH meter |
|---|---|---|---|---|---|---|
| Temperature, °C | 37.5-37.5 | 37.5-37.6 | 37.5-37.6 | 37.5-37.5 | 37.5-37.6 | 37.5-37.6 |
| pH (4.0) | 4.02-4.05 | 4.02-4.06 | 4.01-4.04 | 4.03-4.05 | 4.03-4.10 | 4.01-4.03 |
| pH (7.0) | 7.01-7.05 | 7.01-7.03 | 7.00-7.02 | 7.01-7.03 | 7.02-7.07 | 7.01-7.03 |
| pH (10.0) | 10.01-10.04 | 9.98-10.03 | 10.00-10.02 | 10.01-10.04 | 9.99-10.07 | 10.01-10.03 |
| * Five synchronous measurements were made and the lowest and highest values (minimum-maximum) were given. |
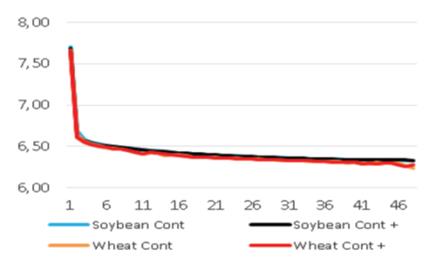
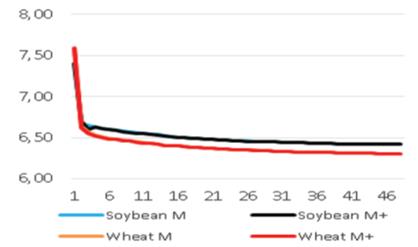
In vitro rumen pH and temperature data (total 192 measurements) were regularly transferred for 48 hours (incubation period). As expected, the pH values decreased with time. The initial pH value (7.71) was continuously decreased to lower value (6.33) after 48 hours. The same trend occurred in all boluses. The temperatures were determined between 38.7 and 38.8°C. However, the same trend can not be expected in living animals due to the fact that animals drink water and also ruminate continuously and so rumen is continuously buffered.
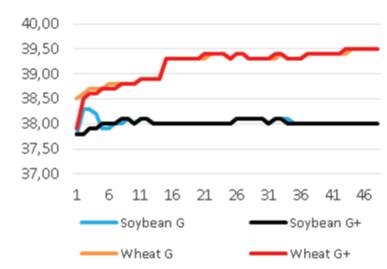
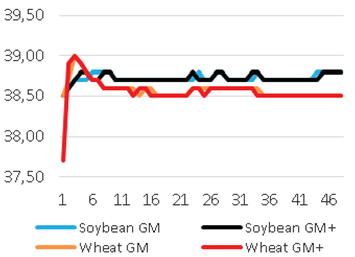
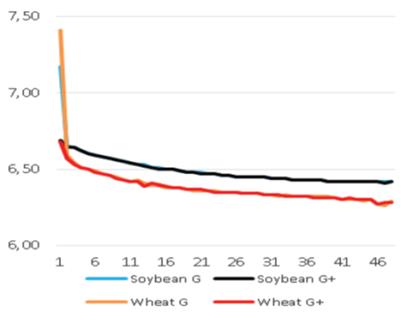
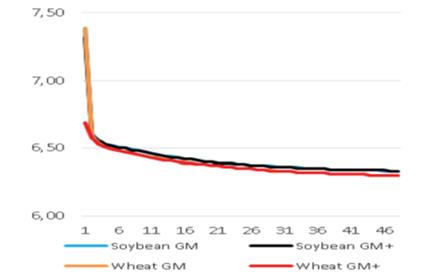
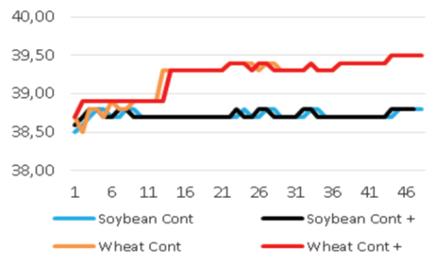
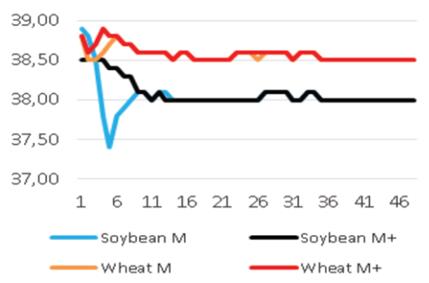
Added groups. In vitro rumen temperatures were lower for soybean straws compared to those for wheat straws (p<0.001). However, sepiolite addition did not affect rumen pH in all treatments for each straw. The lowest rumen temperatures were determined for guar meal added and guar meal+molasses added wheat and soybean straws. This might be attributed to increase in rumen pH. The guar meal addition led to increase in in vitro rumen pH (Figure 1 and 2).
DISCUSSION
The chemical compositions of samples are in agreement with those of Wang et al 14 and Mohamoud Abdi 15. The sepiolite addition led to decrease in CP content of pelleted straws as reported by Gulecyuz 16.
Gaughan 3 compared the WRS with manual pH measurements and then he reported that ruminal pH values measuremented with WRS were between 5.48-5.77 in concentrates, 7.18-7.28 in hays and mixed feeds (hay/concentrates) were between 6.22-6.85. Phillips et al 6, reported that manual pH measurements (average 6.64) were similar to pH values (7.03) determined by using boluses. Dado and Allen 17, Penner et al 18, Penner et al 19 and Phillips et al 6, reported a high correlation (r2 =0.85-0.95) between manual pH measurements and those determined by using boluses. These findings are in agreement with our findings. However, it was reported that pH measurements by boluses might be differed at different hours of a day 3,13,20,21 and also that there might be significant differences between pH measurements determined manually and those determine by using boluses. These differences might be attributed to the differences in rumen fluid sampling techniques or standart pH meters 9.
The pH and temperature values for 48 hours incubation period are given in Table 3 and Figure 1-8. Sepiolite addition did not affect pH in all treatments. Wheat straws showed lowest ruminal pH value. Control groups and molasses added soybean straws and wheat straws were similar in terms of in vitro rumen pH values (Figure 3-6). Guar meal and guar meal+molasses addition increased pH value in soybean straws. This might be caused by addition of guar meal which is a high protein source. Proteinous feeds (compounds) increase rumen pH via their contribution in ruminal ammonia synthesis 22.
Table 3 Average in vitro pH and temperature changes for 48 hour incubation determined by using wireless rumen sensors
| Feed | Average pH | Min.-Max. pH | Averagae temperature, °C |
|---|---|---|---|
| Wheat Cont- | 6.40 ± 0.03d | 6.24-7.67 | 39.23 ± 0.04a |
| Wheat Cont+ | 6.40 ± 0.03d | 6.26-7.66 | 39.24 ± 0.03a |
| Soybean Cont- | 6.43 ± 0.03abcd | 6.33-7.71 | 38.72 ± 0.01b |
| Soybean Cont+ | 6.43 ± 0.03bcd | 6.33-7.69 | 38.72 ± 0.01b |
| Wheat M- | 6.39 ± 0.02d | 6.30-7.39 | 39.19 ± 0.04a |
| Wheat M+ | 6.38 ± 0.01d | 6.30-6.69 | 39.18 ± 0.05a |
| Soybean M- | 6.42 ± 0.02cd | 6.33-7.39 | 38.73 ± 0.01b |
| Soybean M+ | 6.42 ± 0.02cd | 6.33-7.32 | 38.73 ± 0.01b |
| Wheat G- | 6.40 ± 0.03d | 6.30-7.59 | 38.56 ± 0.01c |
| Wheat G+ | 6.40 ± 0.03d | 6.30-7.58 | 38.58 ± 0.01c |
| Soybean G- | 6.51 ± 0.02a | 6.42-7.39 | 38.04 ± 0.03de |
| Soybean G+ | w6.50 ± 0.02a | 6.42-7.40 | 38.09 ± 0.02d |
| Wheat GM- | 6.39 ± 0.02d | 6.26-7.41 | 38.58 ± 0.02c |
| Wheat GM+ | 6.38 ± 0.01d | 6.27-6.68 | 38.56 ± 0.02c |
| Soybean GM- | 6.50 ± 0.02ab | 6.42-7.17 | 38.03 ± 0.01de |
| Soybean GM+ | 6.49 ± 0.01abc | 6.41-6.69 | 38.01 ± 0.01e |
| Significant | <0.001 | <0.001 | |
| Wheat Cont-: wheat control, Wheat Cont+: wheat control with sepiolite, Wheat M-:wheat molasses, Wheat M+: wheat molasses with sepiolite, Wheat G-: wheat guar meal, Wheat G+: wheat guar meal with sepiolite, Wheat GM-: wheat guar meal+molasses, Wheat GM+: wheat guar meal+molasses with sepiolite, Soybean Cont-: soybean control, Soybean Cont+: soybean control with sepiolite, Soybean M-:soybean molasses, Soybean M+: soybean molasses with sepiolite, Soybean G-: soybean guar meal, Soybean G+: soybean guar meal with sepiolite, Soybean GM-: soybean guar meal+molasses, Soybean GM+: soybean guar meal+molasses with sepiolite |
The average pH value determined in present study was found higher than pH value reported by Desnoyers et al 9. This can be attributed to use of different feeds in the two studies. In the present study, use of forage led to increase in rumen pH. Castro-Costa et al 8, monitored the rumen temperature and pH changes in milk goats fed on different rations (70 and 50% forage) and under different climatic conditions (20-23°C and 30-37°C) by using boluses. The researchers found 0.31 unit higher pH values in goats fed on ration with higher forage content (70%) (6.56 vs 6.25) and 0.12 unit lower pH values in goats raised under high ambient temperature (30-37°C) (6.55 vs 6.43). The rumen temperature was found similar for two ration types (38.82°C for high forage ration and 38.92 °C for low forage ration), but it was higher (39.9°C) in goats raised under high temperature (30-37°C) compared to that (39.6°C) in goats raised under normal temperature (20-23°C). These researchers reported that boluses could give reliable results related to rumen pH and temperature and also that the water consumptions could be determined by increase in rumen pH. The average pH values determined in the present study (6.38 and 6.51) were found similar to those reported by Castro-Costa et al 8.
If the ruminal pH become less than 5.75, it will be subacut rumen acidosis 5. But there is no SARA problem in this study for using roughage. The lowest, highest and average rumen pH values in dairy cows were reported as 5.30, 7.39 and 6.28, respectively, by Hanušovský et al 13. Giger-Reverdin et al 23, reported pH values as 6.28 in goats fed on ration with 70:30 forage/concentrate ratio and as 5.96 in those fed on ration with 30:70 forage/concentrate ratio by using boluses. The researchers reported that there was SARA risks in goats fed on concentrate based diet. It seems that, use of rumen sensors has a big potential for diagnosing and preventing SARA. Castro-Costa et al 8, Wahrmund et al 24 and Loholter et al 21 reported that the rumen pH decreased and rumen temperature increased with increasing time after feeding. The same trend was observed in the present study.
Castro-Castro et al 8 determined rumen temperature as 38.9°C by using boluses and they reported that the different diets did not affect the rumen temperature. Alzahal et al 25 and Loholter et al 21 reported that high concentrate diets (40-65%) led to increase in rumen temperature. Heat, which is a by product of microbial fermentation, reaches the peak level more quickly in ruminants consuming high concentrate diets 26. Our findings related to this subject are in agreement with the previous studies.
In in vitro studies, the activities such as rumination and drinking water which strongly affect rumen pH are not taken into account. Therefore, in in vivo studies, ruminal pH value may increase at sometimes. Besides, it can change individual difference of animals 3. This can lead to erroneous pH measurements. In the present study, the data were obtained under controlled conditions. These data might be different from those obtained on live animals because, the locations (rumen, reticulum) at which boluses are located have significant effect on the obtained data 1,7,27. Thus, it is known that different pH values can be determined in rumen and reticulum compartments of an animal in the same day. It is also known that substantial changes can occur in reticulum temperature due to water consumption 4,8. Furthermore, rumen environmental conditions (bacteria colonization etc.) can lead to change in rumen pH. The diurnal ruminal pHs can differ from an animal to another 9. This fact indicates that the in vitro method used in the present study is a promising method due to its reliability and ease of application. Our findings show that the boluses can be integrated to the in vitro systems.
In the study, temperature controlled Daisy incubator was used and temperature change was not allowed. For this reason, the temperature data obtained in the present study were expected to be similar. The existence of differences in temperature data obtained in present study pointout that temperature measurements might be disputable.
In conclusion, it is expected that WRSs will have widespread use in near future. These sensors supply information related to the rumen conditions of experimental animals. The sensors which can adapt to many research programmes have an ease of application in rumen fistulated animals. Furthermore, they remove the need to collect rumen fluid 7. The present study showed that the pH and temperature data can be obtained at willed time intervals. Thus, it is possible to determine the effects of feeds and treatments on rumen fermentation by using the data obtained from the boluses. The short battery lifes (nearly 3-4 months) and high prices prevent common use of boluses. For this reason, further researches should be conducted for developing low-cost and long-lived boluses in future. Furthermore, it might be possible to determine the contents of volatile fatty acids, ammonia and methane in rumen by using boluses. More detailed in vitro and in vivo researches are needed in this topic.