Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
CT&F - Ciencia, Tecnología y Futuro
Print version ISSN 0122-5383On-line version ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro vol.3 no.1 Bucaramanga Jan./Dec. 2005
ABSTRACT
The characterization of naphtha obtained by direct distillation of medium and heavy crude oils is often limited by the low yield of these fractions. Gas chromatography is a technique that allows a complete determination of the chemical composition of these fraction. However, the prediction of properties such as octane rating and RVP from chromatographic data is a difficult task because there are not adequate models to predict the interaction of the different components, and particularly in the case of heavier fractions, there are some problems for the complete separation of components under the gas chromatographic conditions. The IR technology constitutes a rapid and effective tool to predict several properties of naphtha from the correlation of the spectrum in the infrared area and the properties. In this study, prediction models were developed in a Petrospec Cetane 2000 analyzer, in order to predict in a fast and simple way, the density, the antiknock index and the aromatic content of straight run naphtha obtained in a standard crude oil distillation unit. The equipment used was designed in the factory for the exclusive characterization of medium distillation and not for lighter fractions therefore this work constitutes an innovation given the extensive applications of this type of analyzers.
Keywords: chemometrics, infrared spectrophotometry, infrared analyzer, petroleum naphthas, octane rating of naphthas.
RESUMEN
La caracterización de naftas obtenidas por destilación directa de crudos medios y pesados a menudo se ve limitada por el bajo rendimiento de dichas fracciones. La cromatografía de gases es una técnica que permite determinar la composición química detallada de estas fracciones pero la predicción de propiedades como el octanaje y el RVP a partir de los datos cromatográficos es una tarea difícil pues no se cuenta con modelos adecuados para predecir las interacciones de los diversos componentes y, especialmente en el caso de la fracciones más pesadas, se presentan dificultades en la separación completa de componentes en las condiciones cromatográficas. La tecnología IR constituye una herramienta rápida y efectiva para predecir varias propiedades de una nafta a partir de la correlación del espectro en la región del infrarojo y las propiedades. En el presente estudio se desarrollaron modelos predictivos en un analizador Petrospec Cetane 2000 para predecir en forma rápida y sencilla la densidad, el índice antidetonante y el contenido de aromáticos de naftas de destilación directa de crudos. El equipo utilizado es diseñado en fábrica para caracterizar exclusivamente destilados medios y no fracciones más livianas por lo cual, este trabajo constituye una innovación dado que amplía las aplicaciones de este tipo de analizadores.
Palabras clave: quimiometría, espectrofotometría infraroja, analizador infrarojo, naftas del petróleo, octanaje de naftas.
INTRODUCCÍON
For the complete characterization of a crude oil it is necessary to measure several physical-chemical properties of the whole crude oil and every cut obtained by direct distillation of it under standard conditions.
Light crude oils (API > 28) typically present a high yield of naphtha which allows the complete chemical characterization of these fractions. Medium and heavy crude oils have a lower yield in their lighter cuts, which constitutes an important limitation for the chemical characterization of those fractions.
The chemical composition of naphtha is typically determined by gas chromatography with capillary column or multi-columns equipment with valves (Beens et al., 2003). The chemical composition of naphtha makes it possible to obtain approximate values of the RON and MON octane rating of it, using statistical methods developed for that purpose (Anderson et al.,1972; Crawford and Hellmuth, 1990). However, the accuracy of this prediction depends on the detailed knowledge of the interactions of the different compounds present in the naphtha. Even though, many of the gas chromatograps dedicated to the analysis of gasoline include in their software the facility to calculate the antiknock index from the chemical composition. The accuracy of the predicted values in some cases is far from ideal, and therefore it is necessary to find other technologies in order to make a better estimate of this property.
In addition to the above, it is worth mentioning, that the heavier the cut of naphtha the lesser the accuracy of the analysis of the chemical composition obtained by gas chromatography, due to superposition of peaks because of limitations in the resolution of the separation columns.
The NIR technology has been used lately as an effective tool to predict the properties of fuels and some companies have developed methods for simultaneous prediction of several properties of gasoline, including among others, chemical composition, octane rating, RVP and distillation curve (Bohács et al., 1998). Likewise, works has been performed for predicting the properties of fractions that are the feedstock for catalytic reforming process, which are normally developed in NIR equipments with Fourier Transform. On this subject there are powerful applications reported, that allow the prediction of the results of PIANO analysis in this type of matrixes (Chung et al., 1999).
The near infrared area which covers a range of 12800 to 4000 cm-1 shows absorption bands which correspond to overtones and combinations of fundamental C - H, O - H and N - H vibrations because of the large anharmonicity of those vibrations involving the light hydrogen atoms. Consequently a spectrum is expected to be much simpler than a conventional mid IR region (4000-400 cm-1) spectrum. Given that the overtones and the combination bands are much weaker than the fundamental bands (usually by a factor of 10 to 100), the near infrared spectroscopy (NIR) allows the analysis of samples up to several millimeters thick (Bokobza, 1998). The great development of the NIR technology is associated to the current developments of optical fiber which enables the transmission of optical data information efficiently, and therefore has been associated with the development of on-line process analyzers.
Notwithstanding the above, the spectroscopy in the medium infrared contains more analytical information based on which it is possible to develop stronger predictive models. The Cetane 2000 was developed based on this; it is an equipment that operates by using filters, most of which operate in the middle infrared region. This allows the equipment to work with small optic path (200 micrometers) and volume samples as low as 20 ml (Croudace, 2001).
The machine uses the Petrospec R software that allows to develop all the calibration models. The calibration models used by the PetroSpec Cetane 2000 instrument are calculated using the mathematical procedure called multi-linear regression (MLR) analysis. The models have the form:
(1)
Px = M0 + M1*F1 + M2*F2 + ... + Mz*Fz
Where:
Px is the component concentration or value for property x
Fz is the absorbance value obtained from filter z
Mz is the parameter estimate for filter z calculated using MLR analysis.
M0 is the intercept for the model.
The Mz and M0 values constitute the calibration model used for predicting the parameter Px for a sample using the absorbance data Fz. To calculate the calibration model, an equation is written for each sample in the calibration set by substituting the component concentration or property value for Px (the dependent variable) and the absorbance values for Fx (the independent variables). The MLR analysis is used to calculate the values for Mz and M0 that represent the best solution for the set of calibration equations. The best solution is obtained by minimizing the difference between the Px values obtained using standard methods (observed value) and the Px values obtained by substituting the Mz, M0, and Fz values into Equation 1 and solving for Px (estimated value).
The Mz and M0 values are substituted into an equation of the form described in Equation 1. An estimate of the property value is calculated by substituting the absorbance data, Fz, from a sample into the equation for the chemical concentration or property value. There is one equation for each chemical concentration or property value linked to a trigger.
A calibration model is used to transform spectroscopic data acquired from a sample into a prediction of a physical property value or component concentration for the sample. In the application of the calibration model, the instrument acquires the infrared absorption spectrum of the sample, and the mathematical model stored in the computer memory is used to convert the spectral data into a property value. A unique model is used to predict each physical property value and chemical concentration.
This study evaluates the applicability of this analyzer in the characterization of straight run naphthas.
EXPERIMENTAL PART
For this study it was used a Cetane 2000 analyzer with Petrospec R software. The optical system of this equipment consists of 14 filters and the pathlength is 200 micrometers.
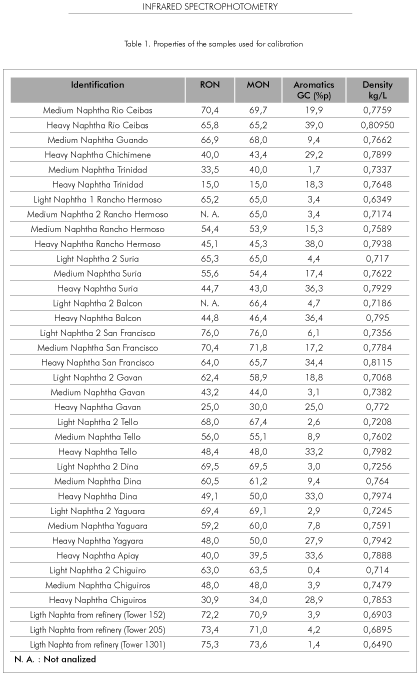
All the samples referenced in Table 1 were selected as the calibration set and were read as standards in the Cetane 2000 using a pumping time of 60 seconds. Most of these samples were obtained by direct distillation of crudes in one atmospheric distillation unit operated according the ASTM D2882 standard. The chemical composition of the naphtha samples were determined in an Agilent 6890 chromatograph provided with a capillary column of fused silica, 100 m long and 0,5 um wide. The compositional analysis software used was Hydrocarbon Expert of Separation Systems. The density of the samples was determined by the ASTM D 4052 method. The octane rating of the reference samples was determined by the following methods: ASTM D2699 (RON) and ASTM D2700 (MON).
With the objective of assembling a robust model, the samples comprising the calibration set were selected with the idea of having sufficient variability in the concentration of the chemical components to cover the range expected to be present in the samples to be analyzed.
RESULTS
Table 1 summarizes the type and properties of the samples used to develop the predictive models. Most of the naphtha samples used for the calibration of the equipment were medium and heavy naphtha obtained in the distillation lab from Colombian crude oils. Very few samples of light naphtha could be used in the training set. To have more light samples in the calibration set there were included some light naphthas obtained directly in the refinery distillation units.

As expected, for any given naphtha the RON and MON octane ratings are very close to each other. Generally speaking, as the naphtha becomes heavier the octane rating becomes lower and the aromatic content becomes higher.
Figure 1, shows how some of the infrared spectra of naphthas used for the calibration, look like on the detector of the Cetane 2000 equipment. All the observed differences in these spectra were used to develop the models that correlate a defined property with the spectra using the petrospec R software incorporated in the equipment.

Figure 1. Infrared spectra of some naphthas obtained in the cetane 2000
Figure 2, 3, 4 and 5, show the good linearity found between the measured property and the value predicted from the respective IR spectrum of the sample. These results indicate that the quality of the predictive models is good in all the cases.

Figure 2. Correlation between experimental density and value predicted by the IR model that was developed

Figure 3. Correlation between experimental ron octane rating and value predicted by the IR model that was developed

Figure 4. Correlation between experimental mon octane rating and value predicted by the IR model that was developed

Figure 5. Correlation between the experimental aromatic content and the predicted value by the developed IR model
Table 2 summarizes the parameters obtained with the calibration data set that define the statistical performance of each of the predictive models developed herein.
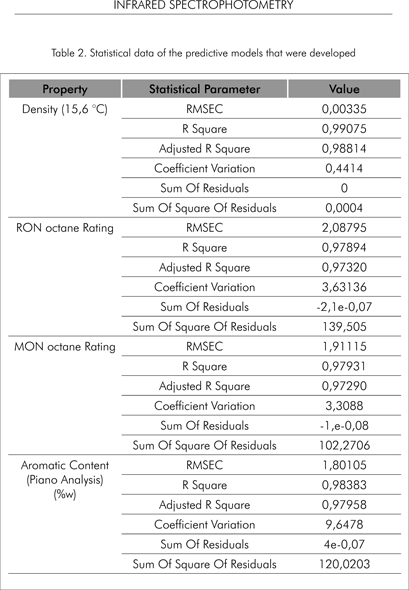
All the results show the quality of the values that may be obtained with the predictive models that were developed.
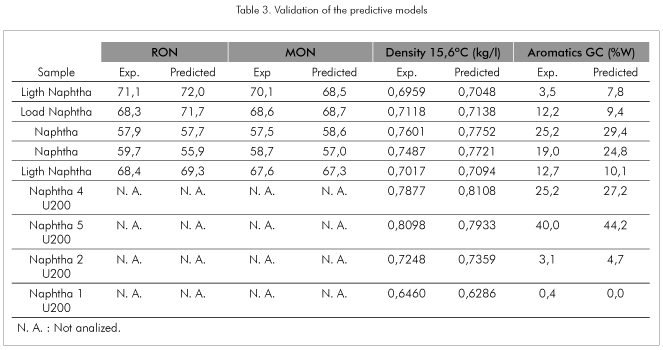
Table 3 resumes some of the data obtained with samples selected for the model validation step. The samples considered in this set cover the whole range from light to heavy naphthas and also there were included samples of straight run naphthas coming directly from the refinery distillation units. The table summarizes the experimental and the predicted value for the evaluated properties.
The performance of the models are usually measured by the root mean square error of prediction (RMSEP), calculated as (Celio, 2003):
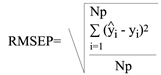
Where Yi and yi are, respectively, the predicted and reference values of the concentration/property for the validation set of Np number of samples. Table 4 summarizes the RMSEP of all the properties predicted. As expected, the prediction errors are a little higher than the calibration errors but considering the speed of the analysis and the limited number of samples included in the calibration set, the predicted data could be considered good.

CONCLUSIONS
• The evaluated technology could be applied for predicting in a fast and simple way the proximate values of octane rating (RON and MON), density, and aromatic content for straight run naphthas.
• The method developed constitutes an important contribution to the characterization of naphthas from crudes with low volumetric yield of light fractions.
• Compared to the standard test methods, the proprosed methodology reduces substantially not only the analysis costs, but the required sample volume and the analysis time.
• The developed method constitutes and innovative extension of the applications of the Cetane 2000 analyzer, an equipment dedicated to diesel fuel analysis.
ACKNOWLEGMENTS
The authors would like to thank to the Laboratory and Pilot Plant Coordination Area of the Instituto Colombiano del Petróleo (ICP) for the supply and characterization and of all the samples used for this work and also for the reading of all these samples in the analyzer. Also we want to thank to the Maintenance Department people for the optimization of the pumping system of the analyzer.
BIBLIOGRAPHY
1. Anderson, C., Sharkey, J. M. and Walsh, R. P., 1972. “Calculation of the research octane number of motor gasolines from gas chromatographic data and a new approach to motor gasoline quality control. J. Inst. Petroleum, 58, 83-94. [ Links ]
2. Beens, J., Feuerhelm, J.T., Fröhling, J.C., Watt, J. and Schaatsbergen, G., 2003. “A Comparison of Ten Different Methods for the Analysis of Saturates, Olefins, Benzene, Total Aromatics, and Oxygenates in Finished Gasolines. Journal of Chromatographic Scien., 41 (10): 564-571. [ Links ]
3. Bohács, G., Ovádi, Z. and Salgó, A., 1998. “Prediction of gasoline properties with near infrared Spectroscopy. J. Near Infrared Spectroscopy, 6, 341-348. [ Links ]
4. Bokobza, L., 1998. “Near Infrared Spectroscopy. J. Near Infrared Spectroscopy, 6, 3-17. [ Links ]
5. Celio, P., 2003. "Near infrared Spectroscopy: fundamentals, practical aspects and Analytical applications". J. Braz. Chem. Soc., 14 (2). [ Links ]
6. Crawford, N. R. and Hellmuth, W., 1990. “Refinery octane blend modelling using principal components regression of gas chromatographic data. Fuel, 69, 443-447. [ Links ]
7. Croudace, M., 2001. Analysis of fuel properties by Mid-Infared Spectroscopy. Word Refinery. [ Links ]
8. Hoeil, C., Joon, S. and Min-Sik-Ku., 1999. “Detailed Compositional of Naphtha and Reformate by Near Infraed Spectroscopy". Application Note. American Laboratory. 24-25. [ Links ]














