Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Boletín de Investigaciones Marinas y Costeras - INVEMAR
Print version ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.28 no.1 Santa Marta Jan./Dec. 1999
INFLUENCE OF DIFFERENT DIETS ON LENGTH AND BIOMASS PRODUCTION OF BRINE SHRIMP ARTEMIA FRANCISCANA (KELLOG, 1906)A.
INFLUENCIA DE DIFERENTES DIETAS EN LA LONGITUD Y LA PRODUCCIÓN EN BIOMASA DE LA ARTEMIA FRANCISCANA.
Manuel García-Ulloa Gómez1, Julián Gamboa Delgado1 , José Luis Zavala Aguirre2, Tetsuya Ogura Fujii3 and Patrick Lavens4.
1 Universidad Autónoma de Guadalajara, Laboratorio de Ciencias Marinas, Barra de Navidad Ap. Post. 3, Barra de Navidad, Jalisco, México; C.P. 48987. e-mail: manuelgu@uagunix.gdl.uag.mx (M.G.U.G. y J.G.D.).
2 Universidad Autónoma de Guadalajara, Facultad de Ciencias Naturales y Agropecuarias (J.L.Z.A).
3 Universidad Autónoma de Guadalajara, Facultad de Ciencias Químicas (T.O.F.).
4 University of Gent, Laboratory of Aquaculture, Belgium (P.L.).
ABSTRACT
Total length and biomass production of the brine shrimp Artemia franciscana were studied fed on soybean and wheat micropulverized meals (applied alone or mixed at different proportions), live microalgae (Tetraselmis suecica and Chaetoceros calcitrans), and dried Spirulina as diets. Eight diets were tested in triplicates during 10 days. Significant differences (P < 0.05) were observed from day 1 onwards. The mixed meal-based diets showed better production results. At day 1, the Artemia nauplii fed on the 70% wheat meal/ 30% soya meal diet were 30% longer compared to the animals from the C. calcitrans group. At day 10, the organisms fed with the 100% soya meal diet were 68% longer than those fed on the C. calcitrans diet. The final biomass production (wet and dry weight) for the mixed meal diet groups was higher than that obtained for the algal treatments, although survival rate was higher for the C. calcitrans diet. A soya-wheat meal diet is recommended for brine shrimp biomass production.
KEY WORDS: Artemia franciscana, soya meal, wheat meal, microalgae, length, biomass production.
RESUMEN
Se evaluó la longitud total y la producción de biomasa de Artemia franciscana aplicando harinas micropulverizadas de soya y trigo (como ración única o mezcladas), microalgas vivas (Tetraselmis suecica y Chaetoceros calcitrans) y Spirulina seca como dietas experimentales. 8 dietas fueron examinadas por triplicado durante 10 días. Se observaron diferencias significativas (P < 0.05) entre los tratamientos desde el primer día. Las dietas de harinas mezcladas mostraron los mejores resultados en longitud. Al primer día, las larvas de A. franciscana alimentadas con 70% harina de trigo/30% harina de soya fueron 30% más grandes comparados con los animales del grupo alimentados con C. calcitrans. Al día 10, los organismos que recibieron una dieta con 100% harina de soya, fueron 68% más grandes que los del grupo de C. calcitrans. La producción final de biomasa (peso seco y húmedo) para las dietas mezcladas fue mayor que la obtenida para los grupos de microalgas (viva o seca), aunque la sobrevivancia mayor fue observada en el grupo de C. calcitrans. Se recomienda el uso de la harina de trigo mezclada con la harina de soya para la producción de biomasa de A. franciscana.
PALABRAS CLAVE: Artemia franciscana, harina de soya, harina de trigo, microalgas, longitud, producción de biomasa.
INTRODUCTION
It is well accepted that Artemia is the most widespread live food item used in the production of shrimp, prawn and fish larval stages. It can be used in different forms in hatcheries and nurseries, e.g. decapsulated cysts, nauplii, metanauplii, juvenile and adult stages, frozen and freeze-dried Artemia biomass. Artemia biomass is nowadays more frequently used for specific stages of aquaculture species as it enhances production characteristics and overall stress resistance (e.g. in penaeid shrimp nurseries in China, and in Atlantic halibut hatchery production), and/or decreases cannibalism in dolphin fish and lobster larviculture (Lavens and Sorgeloos, 1991). Recent work also report that adult boosted Artemia is added to the shrimp brood stock maturation diet to induce moulting and spawning of the marine white shrimp (Naessens et al., 1995). Due to its particular biological characteristics Artemia can be fed on different diets, from live microalgae to microcapsules and waste products from the food industry (Lavens and Sorgeloos, 1991). Coutteau et al. (1990) found a better growth and survival of the brine shrimp fed on enzymatically-treated yeast after removing the yeast cell wall making it more digestible.
The use of waste products from the food industry (such as rice bran, corn bran, soybean meal, lactoserum and others) is recommended by Lavens and Sorgeloos (1991), because of its low cost and worldwide availability. Thus, the goal of the present study is to evaluate the effect of different dietary sources (agriculture by-products, dry and live microalgae) on the length and Artemia biomass production.
MATERIALS AND METHODS
Artemia origin and culture conditions
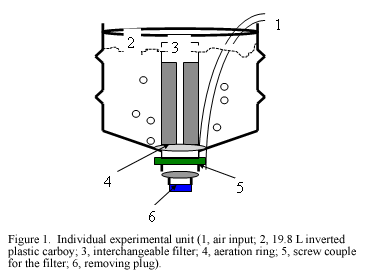
Artemia cysts were obtained from a commercial label (GREAT LAKE ARTEMIA™, Zions, “A” degree, Salt Lake City, Utah, USA) and hatched according the standard methodology proposed by Sorgeloos et al. (1987). Brine shrimp nauplii were experimentally kept under the following culture conditions: 25±2.5oC water temperature, 33±1.3 ppt salinity, 8.0±0.4 pH, > 5 mg L-1 dissolved oxygen, and 13L:11D photoperiod. Twenty four 15 l plastic carboys were used as open system individual culture containers (Figure 1). These were fitted with a central filter and an aeration ring at the bottom. Seawater was filtered through sand and cartridge filters up to 5 µm and sterilized with UV light before entering to the culture system. Culture was subjected to a daily renewal rate of 100% of the volume from day 1 to day 4. From day 5 onwards, the total water volume was exchanged twice a day to keep good water conditions. Initial Artemia density was adjusted at 2,500 nauplii per liter.
Diets and dietary concentrations
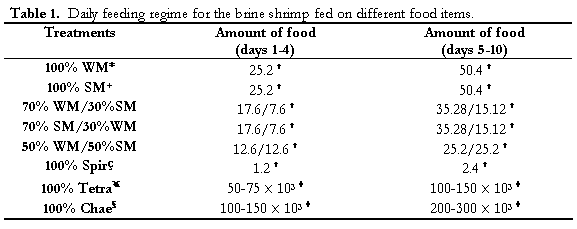
Live microalgae (Tetraselmis suecica and Chaetoceros calcitrans) were cultured using the f/2 culture medium (Guillard, 1975) and kept semi-continuously (Ukeles, 1973) in 19.8 l glass carboys from which a partial volume was daily extracted (at log phase) to feed the animals until the end of the experiment. Spirulina dried powder (CYANOTECH CORPORATION™, Hawaii, USA) was daily weighed and mixed in seawater to get a concentrated solution according to the daily dietary dosage. Both soya and wheat meals (based solely in the small thin bark) were obtained from an agriculture farm, micropulverized and homogenized in seawater, and passed through a 50 µm mesh size sieve in order to get the adequate dietary size particle for Artemia (Bengston et al., 1991). Experimental diets were: 100% soya meal (100SM), 100% wheat meal (100WM), 70% wheat meal/30% soya meal (70WM/30SM), 70% soya meal/30% wheat meal (70SM/30WM), 50% wheat meal/50% soya meal (50WM/50SM), 100% T. suecica (Tetra), 100% C. calcitrans (Chaet) and 100% dried Spirulina sp. (Spir). Total daily dietary dosages were distributed in three rations (at 9:00, 13:00 and 18:00 hrs everyday). (Table 1) shows the daily feeding regimes for each food item. The meals and the Spir dietary concentrations were adjusted by using a secchi disk for measuring the water turbidity (Bossuyt and Sorgeloos, 1980) no less than 30-35 cm depth according to previous experimental observations carried out in our lab, which also included gut content observations of Artemia. For the live microalgae, the cell concentrations were adjusted by applying the same turbidity criterion. In this case, the number of cells required to reach the 30-35 cm water transparency is shown in (Table 1).
The different treatments were tested in triplicates. In the case of the live microalgae, daily dosages at 13:00 and 18:00 hours were kept at 4oC in order to reduce their metabolism and keep them at the proper concentration for feeding the animals at those times.
Evaluation
Daily, culture parameters and survival (expressed as the porcentage of the final Artemia density/ initial Artemia density; Cruz et al., 1993) were obtained and ten animals were taken out from each container (30 per treatment) and measured (from the naupliar eye to the telson; Amat, 1980) using a BAUSH & LOMBâ (USA) reflexion microscope. Routine observations were also recorded every day (gut content, motility, parasites, etc). The experiment was finished once the Artemia sexual characteristics were observed, given mainly by the transformation in claspers of the second pair of antenae in males (Sorgeloos et al., 1986). At the end of the experiment (day 10), the total wet biomass from each treatment was collected on a 250 mm mesh size sieve and the excess of water was removed by means of absorbent paper. Afterwards, the samples were dried (48 hrs at 60oC) and the dry weight was also registered. A one-way analysis of variance was applied to compare the results. Significant differences in means among treatments were determined using Tukey’s multiple range test.
RESULTS
Daily mean total length per treatment is shown in (Table 2). Significant differences (P < 0.05) were detected from day 1 onwards. The diets in which meals were mixed displayed the biggest body length after 24 hours in culture, being 998, 983 and 962 mm for the 70WM/30SM, 70SM/30WM and 50SM/50WM groups respectively. For these groups, length values at the end of the experiment (day 10) registered an increase of around thirteen-fold regarding to the initial length value (493 mm).
On the other hand, the Chaet treatment gathered the lowest length at day 10 (2,186 mm), meanwhile the animals belonging to the Tetra and Spir diets showed an increase of aproximately ten times bigger (4,867 and 4,770 mm resp.) in relation to the initial value. The largest animals were observed when wheat and soya meals (at any concentration) were included in the diet.
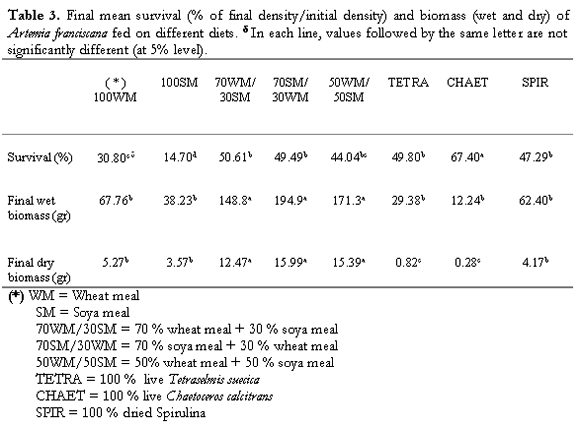
For the final mean survival (Table 3), the 100SM and 100WM groups registered the lowest values (14.7 and 30.8 % resp.), but their final total wet weight was higher than those obtained when feeding live algae. The mixed meal diets showed the highest wet and dry biomass productions. In this case, animals from the 70SM/30WM group shown the highest values (149.9 and 15.99 gr for the wet and dry weight resp.). Although the Chaet group obtained the highest survival value (67.4 %), its final wet biomass reached only 12.24 gr
DISCUSSION
Both microparticles and microcapsules diets are worldwide used as food for shrimp and prawn larvae (Koshio et al., 1989; Jones et al., 1993; Person Le Ruyet et al., 1993), or for bivalve juveniles (Coutteau and Sorgeloos, 1992). In the case of the brine shrimp, its biological characteristics (continuous, non-selective and particle-feeding organism, Lavens and Sorgeloos, 1991) allow the application of inert diets such as agriculture by-products like soya meal, wheat meal corn meal and others.
The present study showed that the meal diets had a better perfomance on the Artemia growth. Animals fed on meals grew more than 6 mm in 10 days, whereas the algae food based diets (live and dried microalgae) did not exceed 5 mm in the same period. Similar results were observed by Vanhaecke and Sorgeloos (1980; 1989) for different Artemia strains cultured under standard laboratory conditions fed on rice bran meal for a period of 7 days. For variuos brine shrimp strains, they observed that the rice bran meal group registered the highest Artemia length values compared with a diet based on the microalga Dunaliella. Another remarkable observation in our experiment was the fact that the brine shrimp sexual characteristics were observed in the animals belonging to the meal diets from day 7 onwards. Only a small amount of adult animals from the total Artemia population fed on the Tetra and Spir diets, was observed just until the end of the experiment.
For the 100SM and 100WM groups, survival values were the lowest maybe due to the inadequate biochemical composition of the individual diets (Brisset et al., 1982). Iwata (1981) reported a protein content lower than 13% for the wheat and soya bark, which could be reflected as a nutritional deficiency. Lavens and Sorgeloos (1991) recommended the use of a mixture of 2 ingredients to get optimal results for application in intensive Artemia production.
It seems the air supply in the experimental units was not well designed to keep the meal microparticles in suspension when the feeding dosages were doubled (day 4). Sorgeloos (1973) suggested that when culturing Artemia at high densities, the aereation system must be able to properly suspend the feeding particles in the water column to avoid high mortalities, situation which was observed in this experiment mainly in the both 100SM and 100WM diets in which a lot of particles were attached to the culture container surface, deterioring consequently the water quality. Despite the Chae group displayed the highest mean survival value, the length size for the animals belonging to this treatment was around three-fold lower than the meal diet groups, a fact that was reflected in a poor biomass production (wet and dry weight) for that algal group. It suggests that different feeding rates were applied -depending on the experimental diets- despite of the transparency parameter that Bossuyt and Sorgeloos (1980) recommend for Artemia intensive production. It is also important to consider the energy content of each diet, since due to the transparency criteria used for adjusting the daily feeding ratio, the amount of food particles for all diets (meals and microalgae) could vary affecting the energy availability for growth and survival. There was not a positive correlation between the survival values and the both wet and dry weights for all the treatments since the different diets used produced a notable size diversity in the animals, which was also observed by García-Ulloa and Gamboa (1997) using different algal diets fed to the rotifer Brachionus plicatilis and by Yúfera et al. (1993) on the same rotifer fed on two different microalgae.
The obtained results in this experiment suggest the use of meal diets to produce higher Artemia biomass than those that could be produced with algal diets under standard culture conditions, and the consequent reduction on the labor-cost for producing microalgae. Besides, we recommend to estimate the daily food requirement to adjust the exact feeding dosage the Artemia needs for their different life stages. Since Lavens and Sorgeloos (1991) reported that the biochemical composition of Artemia fed on agricultural meals is poor in essential components (e.g. fatty acids), the enrichment technique described by Léger et al., (1987) provides a good possibility to improve its biochemical composition just before feeding to the predator organisms.
ACKNOWLEDGMENTS
We would like to express our thanks to the Ing. Acuí. Daniel E. Godínez S. and Mr. Juan Ramón Almada for their technical assistance during the laboratory experiment. Dr. Mauricio Alcocer R. kindly gave us the opportunity to carry out the experiment at the Laboratorio de Ciencias Marinas, and the Universidad Autónoma de Guadalajara gave the financial support. We also would like to mention the Dirección de Investigación (UAG), especially Dr. Rodolfo Casillas and Ms. C. Miriam Alvarez del Castillo for their complete support in our research area.
LITERATURE CITED
1 Amat, F. 1980. Differentiation in Artemia strains from Spain. In: G. Persoone, P. Sorgeloos, O. Roels & E. Jaspers (Eds). The Brine Shrimp Artemia. Morphology, Genetics, Radiobiology, Toxicology. Universa Press, Wetteren, Belgium, Vol. 1. 19-39. [ Links ]
2 Bengtson, D.A.; P. Léger and P. Sorgeloos. 1991. Use of Artemia as a food source for aquaculture, 11: 255-285. In: Browne R.A; P. Sorgeloos and C.N.A. Trotman (Eds).Artemia Biology. CRC Press, Inc., Boca Ratón, Florida, USA, 374 p. [ Links ]
3 Bossuyt, E. and P. Sorgeloos.1980. Technological aspects on the batch culturing of Artemia in high densities. In: G. Persoone; P. Sorgeloos; O. Roels and E. Jaspers (Eds.). The Brine Shrimp Artemia. Ecology, Culturing, Use in Aquaculture. Universa Press, Wetteren, Belgium, Vol. 3. 456 p. [ Links ]
4 Coutteau, P. and P. Sorgeloos. 1992. The use of algal substitutes and the requirement for live algae in the hatchery and nurseryrearing of bivalve molllusks: An international survey. J. Shelf. Res., 11: 467-476. [ Links ]
5 __________ ; P. Lavens and P. Sorgeloos. 1990. Baker's yeast as a potential substitute for live algae in aquaculture diets: Artemia as a case study. J. World Aquaculture Soc., 21:1-9. [ Links ]
6 Cruz, L.E.; D. Ricque y J.A. Martínez. 1993. Evaluación de dos subproductos de camarón en forma de harina como fuente de proteína en dietas balanceadas para Penaeus vannamei. En: Cruz, E.; D. Ricque y R. Mendoza (Eds). Memorias del Primer Simposium Internacional de Nutrición y Tecnología de Alimentos para Acuacultura. Monterrey, N.L., México. 1993, 205-232. [ Links ]
7 García-Ulloa, M. and J. Gamboa. 1997. Caracterización de una cepa del rotífero Brachionus plicatilis. II. Influencia de diferentes dietas sobre la talla bajo condiciones de cultivo estático. CIMPES (in press). [ Links ]
8 Guillard, R.R. 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith L. and M.H. Chanley (Eds). Culture of Marine Invertebrate Animals. (W.) Plenum, New York, 29-60. [ Links ]
9 Iwata, H. 1981. Introduction to the Chemistry of Food. Fugendo (Ed.), Tokyo, Japan, 983 p. [ Links ]
10 Jones, D.A.; K.M. Salleh and L. Le Vay. 1993. The potential for replacement of live feeds in larval culture. Journal of the World Aquaculture Society, 24:199-210. [ Links ]
11 Koshio, S.; A. Kanasawa; S. Teshima and J.D. Castell. 1989. Nutritional evaluation of crab protein for larval Penaeus japonicus fed microparticulate diets. Aquaculture, 81:145-154. [ Links ]
12 Lavens, P. and P. Sorgeloos. 1991. Production of Artemia in culture tanks.. In: Browne, R.A.; P. Sorgeloos and C.N.A. Trotman (Eds). Artemia biology. CRC press, Inc. Boca Ratón, Florida, USA., 317-350. [ Links ]
13 Léger, P.; D.A.Bengston; K.L. Simpson; P. Sorgeloos and A.D. Beck. 1987. The nutritional value of Artemia: A review. In: P. Sorgeloos; D. A. Bengston; W. Decleir and E. Jaspers (Eds). Artemia Research and its Applications Universea Press, Wetterem, Belgium, 357-372. [ Links ]
14 Naessens, E.; P. Lavens; L. Gómez; C. L. Browdy; K. McGovern-Hopkins; A. H. Spencer; D. Kawahigashi; and P. Sorgeloos. 1995. Maturation performance of Penaeus vannamei co-fed with Artemia biomass. In:Lavens, P.; E. Jaspers and Y. Roetlans (Eds). Larvi ´95 Fish & Shellfish Larviculture Symposium. European Aquaculture Society, Special Publication No. 24, Gent, Belgium. [ Links ]
15 Person Le Ruyet, J.; J.C. Alexandre; L. Thébaud and C. Mugnier. 1993. Marine fish larvae feeding: formulated diets or live prey. Journal of the World Aquaculture Society, 24: 211-224. [ Links ]
16 Sorgeloos, P. 1973. High density culturing of the brine shrimp, Artemia salina. Aquaculture, 1: 385. [ Links ]
17 __________; D.A. Bengston; W. Decleir and E. Jasper. 1987. Ecology, Culturing, Use in Aquaculture. In: Sorgeloos, P.; D. A. Bengston; W. Decleir and E. Jaspers (Eds). Artemia research and its applications. Universa Press, Wetteren, Belgium, 221 p. [ Links ]
18 __________; P. Lavens; P. Léger; W. Tackaert, and D. Versichele. 1986. Manual for the culture and use of brine shrimp Artemia in Aquaculture. Artemia Reference Center, State University of Ghent, Belgium, 319 p. [ Links ]
19 Ukeles, R. 1973. Continuous culture - A method for the production of unicellular algal foods. In: J. Stein (Ed), Handbook of Phycological Methods, Culture Methods and Growth Measurements. Cambridge University Press, London, 233-254. [ Links ]
20 Vanhaecke, P. and P. Sorgeloos. 1980. International study on Artemia IV. The biometrics of Artemia strains from different geographical origin. In: Persoone, G.; P. Sorgeloos; O. Roels and E. Jaspers (Eds). The brine shrimp Artemia Universa Press, Wetteren, Belgium, 393 p. [ Links ]
21 __________ and __________. 1989. International study on Artemia XLVII. The effect of temperature on cyst hatching, larval survival and biomass production for different geographical strains of brine shrimp Artemia spp. Ann. Soc. Zool. Belg., 119: 7. [ Links ]
22 Yúfera, M., E. Pascual and J. Guinea. 1993. Factors influencing the biomass of the rotifer Brachionus plicatilis in culture. Hydrobiología, 255/256:159-164. [ Links ]
DATE RECEIVED: 27/01/1998 DATE ACCEPTED: 07/07/1998