Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería y Universidad
Print version ISSN 0123-2126
Ing. Univ. vol.17 no.1 Bogotá Jan./June 2013
Reactivity Study of Kaolinite and Metakaolinite in Fluoride Media under Hydrothermal Conditions1
Estudio de reactividad de caolinita y metacaolinita en medios fluorurados en condiciones hidrotermales2
Estudo de reatividade de caulinita e metacaulinita em meios fluoretados em condições hidrotermais3
Carlos Alberto Ríos-Reyes4
Craig Denver-Williams5
Óscar Mauricio Castellanos6
1Reception date: June 10th, 2011. Admission date: June 25th, 2012. This article is developed by the Research Group in Basic and Applied Geology, Universidad Industrial de Santander, Bucaramanga, Colombia. This work was supported by the Programme Alban, the European Union Programme of High Level Scholarships for Latin America (Scholarship No. E05D060429CO) and the Universidad Industrial de Santander and the University of Wolverhampton.
2Fecha de recepción: 10 de junio de 2011. Fecha de aceptación: 25 de junio de 2012. Este artículo fue desarrollado por el Grupo de Investigación en Geología Básica y Aplicada, Universidad Industrial de Santander, Bucaramanga, Colombia. Fue apoyado por el Programa Alban, el Programa de la Unión Europea de Becas de Alto Nivel para América Latina (E05D-060429CO) y la Universidad Industrial de Santander y la Universidad de Wolverhampton.
3Data de recepção: 10 de junho de 2011. Data de aprovação: 25 de junho de 2012. Este artigo foi desenvolvido pelo Grupo de Investigación en Geología Básica e Aplicada [Grupo de Pesquisa em Geologia Básica e Aplicada], Universidad Industrial de Santander, Bucaramanga, Colômbia. Projeto apoiado pelo Programa Alban, pelo Programa de la Unión Europea de Becas de Alto Nivel para América Latina (E05D060429CO), pela Universidad Industrial de Santander e pela Universidad de Wolverhampton.
4Geólogo y Especialista en Docencia Universitaria, Universidad Industrial de Santander, Bucaramanga, Colombia. MSc in Geology, Shimane University, Matsue, Japón. PhD in AppliedSciences, University of Wolverhampton, Wolverhampton, Inglaterra. Docente investigador, Escuela de Geología, Universidad Industrial de Santander. E-mail: carios@uis.edu.co.
5BSc, MSc, PhD in Chemist, Salford University, Salford, Inglaterra. Professor, School of Applied Sciences, University of Wolverhampton, Wolverhampton, Inglaterra. E-mail: c.williams@wlv.ac.uk.
6Geólogo, Universidad Industrial de Santander, Bucaramanga, Colombia. MSc in Geology, Shimane University, Matsue, Japón. Docente investigador, Programa de Geología, Universidad de Pamplona, Pamplona, Colombia. E-mail: oscarmca@yahoo.es.
Reception date: June 10th, 2011. Admission date: June 25th, 2012.
Abstract
This paper studies the reactivity of kaolinite and metakaolin in a fluori-nated medium under hydrothermal conditions. This path of conventional zeolite synthesis did not yield promising results, although traces of different zeotypes did crystallize, such as the crystal structures Linde type Y (LTY), zeolite Na-P1 (GIS), chabazite-ca (CHA), sodalite (SOD), cancrinite (CAN) and Linde type A (LTA). Other zeolites reported in this study were zeolite ZSM-5 (MFI type structure) and zeolite ITQ (ISV type structure). The non-reacting starting material and the synthesized products where characterized by x-ray diffraction, scanning electron microscopy, infrared spectroscopy by Fourier transform and thermogravimetric analysis. The products of the synthesis are of interest because they can be used in different industrial applications.
Keywords: Hydrothermal conversion, kaolinite, metakaolin, fluorinated media, zeolitic products.
Resumen
El presente estudio reporta la reactividad de caolinita y metacaolinita en un medio fluorurado bajo condiciones hidrotermales. Esta ruta de síntesis convencional de zeolitas no produjo resultados promisorios, aunque cristalizaron trazas de diferentes zeotipos, como las estructuras cristalinas linde tipo Y (LTY), zeolita NaP1 (GIS), chabazita (CHA), sodalita (SOD), cancrinita (CAN) y linde tipo A (LTA). Otras zeolitas reportadas en este estudio son zeolita ZSM-5 (estructura tipo MFI) y zeolita ITQ (estructura tipo ISV). Los materiales de partida sin reaccionar y los productos sintetizados se caracterizaron por difracción de rayos X, microscopia electrónica de barrido, espectroscopía de infrarrojo por transformada de Fourier y análisis termogravimétrico. Los productos de la síntesis son de interés, ya que pueden utilizarse en diversas aplicaciones industriales.
Palabras clave: Conversión hidrotérmica, caolinita, metacaolinita, medios fluorurados, productos zeolíticos.
Resumo
Este estudo relata a reatividade de caulinita e metacaulinita em um meio fluoretado em condições hidrotermais. Essa rota de síntese convencional de zeólitas não produziu resultados promissores, embora os traços de diferentes zeótipos tenham se cristalizado, como as estruturas cristalinas linde tipo Y (LTY), zeólita Na-P1 (GIS), chabazita (CHA), sodalita (SOD), cancrinita (CAN) e linde tipo A (LTA). Outras zeólitas relatadas neste estudo são zeólita ZSM-5 (estrutura tipo MFI) e zeólita ITQ (estrutura tipo ISV). Os materiais de partida sem reação e os produtos sintetizados caracterizaram-se pela difração de raios X, microscopia eletrônica de varredura, espectroscopia de infravermelho por transformada de Fourier e análise termogravimétrica. Os produtos da síntese são de interesse já que podem ser utilizados em diversas aplicações industriais.
Palavras chave: Conversão hidrotérmica, caulinita, metacaulinita, meios fluoretados, produtos zeolíticos.
Introduction
Since the pioneering work of Barrer in the 1950s the conditions for zeolite synthesis have changed little. Zeolite synthesis has been extensively reviewed in several books and literature on this subject is abundant (Breck, 1974; Barrer, 1982; Jacobs & Martens, 1987; Szoztak, 1998). The general method of zeolite synthesis involves mixing together Si and Al species, metals cations, organics molecules and water, which are then treated hydrothermally and the mixture is then converted into a microporous crystalline aluminosilicate. Most zeolites probably could be obtained at temperatures <100 °C in alkaline solutions. This is generally the case of zeolites with low Si/Al ratios. However, in order to reduce the reaction times (especially for zeolites with high Si/Al ratios) and to control the crystallite sizes, morphologies and compositions, some syntheses are performed at temperatures >100 °C under autogeneous pressure in autoclaves.
Typical precursor materials include: SiO2 and Al2O3 sources, mineralizer and structure directing agents (SDAs). The discovery of SDAs resulted in the preparation of nearly 100 different silicate frameworks (Breck, 1974). The mode of action of these SDAs has been widely investigated by Davies and Zones (1997) and this has led to some empirical rules, without the absolute ability to design new solids (Lobo, Zones & Davis, 1995). Kubota et al. (1996) discussed the properties of the organic cations that lead to the structure-direction of high silica molecular sieves. Zeolites are prepared by hydrothermal crystallization from alkaline reaction mixtures (Breck, 1974; Chezeau et al., 1989; Szostak, 1998), where hydroxide ion (OH-) acts as mineralizer. The fluoride ion (F-) is a unique mineralizer that has only recently been seriously examined for its use in zeolite synthesis (Szostak, 1998).
Flanigen and Patton (1978) were the first to employ fluoride-containing salts in the synthesis of silicalite-1 in 1978. The behavior of the F- is very similar to that of the OH- as a mineralizing agent and a complexing ion and it will also contribute to the formation of the molecular sieve structures (Tavolaro et al., 1992; Rees & Chandrasekhar, 1993; Férona et al., 1994; Koller et al., 1999; Barret et al., 1998; Serrano et al., 2001; Kato et al., 2003; Egeblad et al., 2007). Corma (2004), using F- as mineralizing agent allows one to carry out the synthesis at lower pH than when using OH-, and opens new possibilities, such as the incorporation of elements in the framework which have low solubility in alkaline medium, in order to perform the synthesis in absence of alkaline cations, new possibilities to directly incorporate cations such as NH. + , adequate stability of many possible templates in this medium, expanding the number of potential templates especially when working with concentrated gels. Finally, the synthesis in the presence of F- produces high silica zeolites with a smaller number of defects than in alkaline media and also one has the possibility of growing single crystals large enough for direct structure determination. The use of F- as a mineralizer in zeolite synthesis was developed by Flanigenand co-workers and has resulted in zeolites being prepared with fewer defect sites and higher silica contents. Zeolite synthesis in fluoride-containing media has been studied in connection with the possibility of giving larger zeolite crystals than those without the fluoride species in the synthesis solution and to increase the pH range over which zeolites may be synthesized (Tavolaro et al., 1992; Kim et al., 2004).
Additionally, several groups have reported a catalytic role of fluoride species for silicate hydrolysis and condensation (Schmidt-Winkel et al., 1999). Kim et al. (2004) developed the synthesis of zeolite beta in fluoride media under microwave irradiation as an efficient and fast route of synthesis. The synthesis of crystalline aluminosilicate zeolites can be carried out from clay minerals (Breck, 1974; Barrer, Cole & Sticher, 1968; Boukadir, Bettahar & Derriche, 2002; Klimkiewicz & Drag, 2004; Baccouche, Srasra & Maaoui, 1998; Cañizares et al., 2000). Previous work has shown that the improvement of the properties of the kaolinite by chemical methods is difficult due to its low reactivity. It is not significantly affected by acid or alkaline treatments, even under strong conditions (Lussier, 1991; Murat et al., 1992; Akolekar, Chaffee & Howe, 1997; Perissinotto et al., 1997; Chandrasekhar & Pramada, 1999; Demortier et al., 1999; Xu et al. , 1999). Therefore, kaolinite is usually used after calcination at temperatures between 5 50-950 °C (Mackenzie, 1970) to obtain a more reactive phase (metaka-olinite) under chemical treatments, with the loss of structural water. Several authors have reported the synthesis of kaolinite- and the metakaolinite-based zeolitic materials (e.g., Chorover et al., 2003; Zhao et al., 2004; Lin et al., 2004; Covarrubias et al., 2006; Ríos, Wiliams & Fullen, 2009).
Numerous investigations have been conducted on the stability of kaolinite in high pH conditions (e.g., Bauer & Berger, 1998; Bauer, Welde & Berger, 1998; Cama et al., 2000). Rees and Chandrasekhar (1993) have converted kaolinite and metakaolinite into zeolites by hydrothermal reaction in fluoride media. When a fluoride medium was used, kaolinite gave zeolite-GIS directly without any intermediate metastable phase, whereas zeolite-FAU was the stable intermediate from metakaolinite (Covarrubias et al., 2006).
The aim of this work was to investigate and understand the reactivity of kaolinite and metakaolinite in a fluoride media under hydrothermal conditions synthesis.
1. Experimental
1.1. Materials
The silica and alumina sources used in the synthesis of zeolites were kaolinite and metakaolinite. Well crystallized, fine grained kaolinite (< 2 μπι), Al2SiO5(OH)4, was used as the starting material for zeolite synthesis. It is distributed under the name of Supreme Powder supplied by ECC International. The Metakaolinite (Al2Si2O7) was obtained from calcination of kaolinite. Other reagents used in the activation of the starting materials were: sodium fluoride, NaF (99%, Fluka), ammonium fluoride, NH.F (98 + %, ACS, Aldrich Chemical Company, Inc.), tetrapropylammonium bromide, (CH3CH2CH2)4NBr (98%, Aldrich Chemical Company, Inc.) and distilled water using standard purification methods.
1.2. Synthesis of Zeotypes
Poor crystalline zeotypes have been synthesized after hydrothermal treatment of kaolinite or metakaolinite in fluoride media. The conversion of these raw materials into zeolitic materials was conducted by the conventional hydrothermal synthesis method. The authors used fluoride media, taking into account that - during the synthesis process- fluoride ions act similarly to hydroxyl ions without contributing directly to the pH of the system (Rees & Chandrasekhar, 1993; Houssin, 2003). The fluoride media was prepared by dissolving the activating agents (NaF or NH4F) in distilled water, under stirring conditions in plastic reaction beakers (150-250 ml), in order to obtain fluoride solutions 1.33 and 3.99 M.
The use of different mineralizers is justified by the fact that it is interesting to compare their effect and efficiency in the synthesis process. Calculated amounts of the raw materials were added to the fluoride solution and stirred until the reagents were dissolved and for the homogenization of the reaction mixture. When NH F was used as mineralizer, TPAB was added to the reaction mixture to investigate the formation of silica-rich zeolites in slight acidic media. The progressive addition of reagents was carried out under stirring conditions until dissolve them in order to homogenize the reaction gels. The representative gel molar compositions are depicted in Table 1. The reaction gels were divided in three portions of approximately the same weight, which were transferred to the autoclaves. The crystallization was carried out by hydrothermal synthesis under static conditions in three PTFE (polytetrafluoroethylene = Teflon) bottles (Cowie Technology Ltd) of 65 ml at 100 °C and in teflon lined stainless steel autoclaves of 20 ml at 175 °C for several reaction times. The reactors were removed from the oven at the scheduled times and were quenched in cold water. The pH was measured before and after hydrothermal treatment. After hydrothermal treatment, the reaction mixtures were filtered, washed with distilled water, and the products were dried in an oven at 80 °C. The dried samples were weighted and kept in plastic bags for analysis.
1.3. Characterization
The starting materials and the synthesis products were characterized by several instrumental analysis techniques. Powder X-ray diffraction (XRD) patterns were obtained on a Philips PW1710 diffractometer operating in Bragg-Brentano geometry with Cu-KOC radiation (40 kV and 40 mA) and secondary mono-chromation. Data collection was carried out in the 2θ range 3-50°, with a step size of 0.02°. Phase identification was made by searching the ICDD powder diffraction file database, with the help of Joint Committee on Powder Diffraction Standards (JCPDS) files for inorganic compounds. Fourier transform infrared (FTIR) spectroscopy was carried out using a Mattson Genesis II FT-IR spectrometer in the 4000-400 cm-1 region.
The morphology of the starting material and as-synthesized products was studied with a ZEISS EVO50 scanning electron microscope, under the following analytical conditions: I probe 1 nA, EHT = 20.00 kV, beam current 100 μΑ, Signal A = SE1, WD = 8.0 mm. Thermogravimetric and differential thermal analyses were performed on a Mettler Toledo TG 50 thermobalance. Samples ∼15-20 mg were heated under nitrogen gas flow (20 ml/min) between 25700 oC at a rate of 20 oC/min.
2. Results
2.1. X-Ray Diffraction Analysis
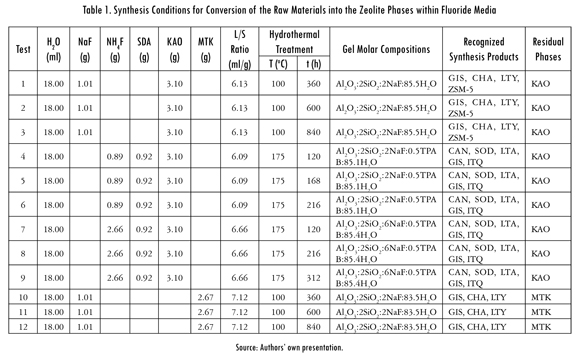
In general, the characterization by X-ray diffraction analysis of the starting materials and the as-synthesized products obtained after their activation in fluoride media reveal that the diffractograms of different products depend on the concentration of the solution used. From the diffractograms of the synthesis products, we highlight the presence of different crystalline phases (in some cases accompanied by an amorphous phase) having crystalline peaks, wider than those corresponding to a single crystalline phase, which may be superimposed to other. Therefore, it is evident the coexistence of crystalline regions, formed by low crystalline zeolitic phases (crystals of small size and probably with structural disorder), which results in relatively wide diffraction peaks, and amorphous regions in some of the resultant products. This is one reason why it is preferable to call these materials as semicrystalline materials. Based on the foregoing, due to their low crystallinity and the width of the peaks, it is particularly difficult to define what type of structure belongs to what the authors referred in the present study as Zeolite ITQ, considering that a refinement was not conducted by the Rietveld method of the structure of the phases present in the resultant products. The XRD patterns of both starting materials and as-synthesized products obtained after hydrothermal treatment of kaolinite and metakaolinite in fluoride media are described below. Figure 1 illustrates the XRD patterns for the results of hydrothermal synthesis from the natural clay in solutions of NaF (1.33 M).
Kaolinite (in red color) is the predominant mineral phase, which can be identified by its characteristic XRD peaks at 12.34° and 24.64° 2θ as reported by Zhao et al. (2004). However, minor impurities, such as illite, muscovite and halloysite, also occur. Metakaolinite (in red color) is characterized by the disappearance of all the XRD peaks of the natural clay, accompanied by the appearance of an amorphous aluminosilicate (see the broad hump at 2θ = 13-33°, having a maximum at 2θ = ∼22o), which according to Ríos (2008), persists between 600 and 950 oC. As can be seen also from the XRD patterns, a slow dissolution of the starting materials, revealed by a progressive reduction of the peak intensity, was accompanied by the appearance of very weak peaks of zeolite Na-P1 (GIS), chabazite (CHA) and Linde Type Y (LTY), which increased in crystallinity with reaction time, although with a large amount of amorphous aluminosilicate material when metakaolinite was used. However, it is also noticed the occurrence of Zeolite Socony Mobil-5 (ZSM-5), widely used in the petroleum industry as a heterogeneous catalyst for hydrocarbon isomerization reactions. However, the synthesis conditions should be optimized in order to obtain highly crystalline zeolites at several hydrothermal treatment time and temperature, and varying molar quantities of water during the hydrothermal treatment step. The adjustment of the starting material composition and synthesis parameters can improve the quality and potential applications of zeolites.
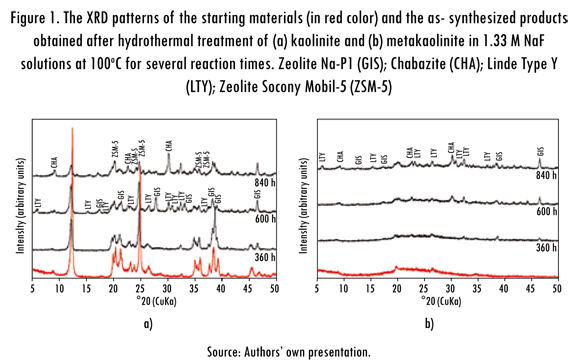
The XRD patterns in Figure 2 show a very slow dissolution of kaolinite and a formation of amorphous aluminosilicate material when low and high concentrations of the mineralizer (NH4F) were used, respectively, and higher temperatures of crystallization. Figure 2a reveals that the synthesis products include zeolite Na-P1 (GIS), cancrinite (CAN), sodalite (SOD) and Linde Type A (LTA). Figure 2b shows the formation of these zeotypes, although with weak peak intensity. However, it is also noticed the occurrence of a zeolite ITQ (structure type ISV), which has been recently obtained by researchers in Valencia (Spain), who succeeded, for the first time, the direct synthesis of zeolite ITQ-43 with mesopores and micropores hierarchically connected, that allowed the reaction of molecules of different sizes. Their results are a breakthrough of great significance in the field of so-called "green chemistry".
According to Jiang et al. (2011), this new zeolite could be used in the processing of crude oil into gasoline and diesel, removing sulfur compounds and nitrogen over, thereby producing cleaner fuels, however, it could also be applied in order to transform natural gas into liquid fuels, to contribute to the production of useful chemicals from biomass, including its potential applications in the field of gas storage, electronics, medicine and perfumery. Therefore, as mentioned before, the synthesis conditions should be optimized to obtain highly crystalline zeolites at several experimental conditions than that used in this study.
2.2. Fourier Transformed Infrared Spectroscopy
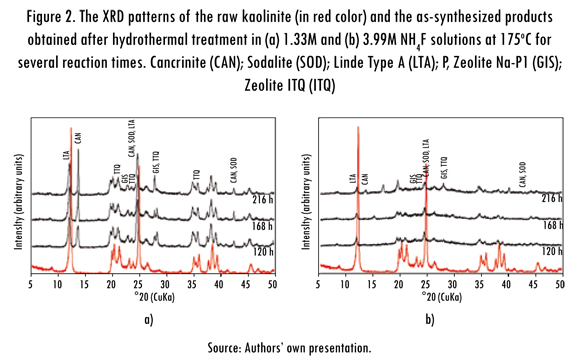
Figure 3 illustrates the FT-IR spectra of the starting materials over the range 400-4000 cm-1. The bands at 3692 and 3619 cm-1 have been assigned to the stretching vibration of hydroxyl groups in the kaolinite structure (Liu et al. , 2001; Saikia et al., 2003; Zhao et al., 2004; Alkan et al., 2005), which are not present in the metakaolinite spectrum. However, the bands at 3669 and 3652 cm cm-1 reported by Zhao et al. (2004) and Hoch & Bandara (2005) were not observed. According to Kristof et al. (1993), the band at 3692 cm-1 represents the stretching vibration modes of inner surface hydroxyls that are located at the surface of octahedral sheets opposite to the tetrahedral oxygens of the adjacent kaolinite layer, whereas the band at 3619 cm-1 is related to the stretching vibration modes of inner hydroxyls and refer to OH groups located in the plane common to the octahedral and tetrahedral sheets.
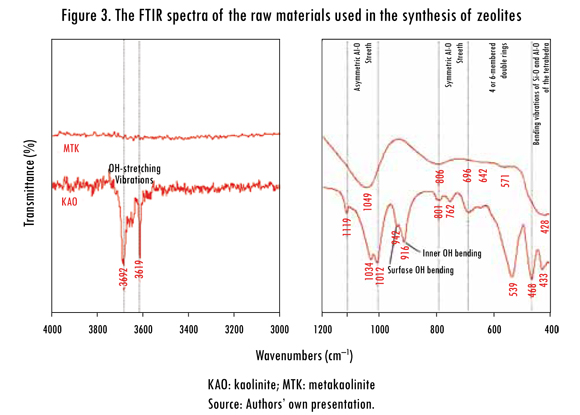
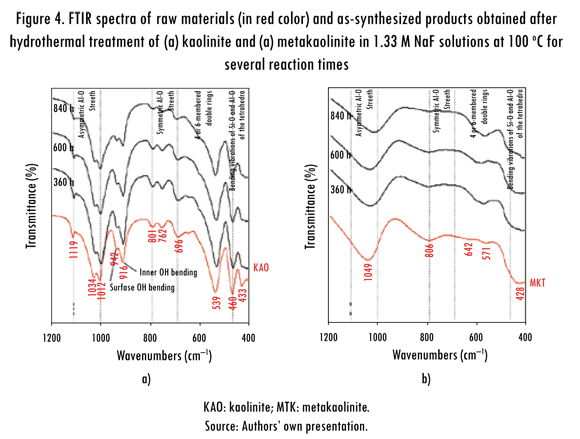
The band at 1119 cm-1 is referred to Si-O stretching vibrations, while the bands at 1034 and 1012 cm-1 are caused by the Si-O-Si and Si-O-Al lattice vibrations (van der Marel and Beutelspacher, 1976). The OH bending vibrations at 942 and 916 cm-1 can be referred to the surface OH bending and inner OH bending (Frost et al., 2002). According to van der Marel and Beutelspacher (1976), these bands are mainly caused by Al-OH groups. Further, bands in low range of frequency (762, 696 and 539 cm-1) can be largely attributed to different Si-O and Al-O vibrations; the first two of them can be attributed to the distortion of the tetrahedral and octahedral layers (Hoch & Bandara, 2005).
The bands at 468 and 433 cm-1 can be attributed to bending vibrations of Si-O and Al-O of the tetrahedra. The conversion of kaolinite to metakaolinite is revealed by the disappearance of these characteristic bands. These changes are similar to those reported in other studies (Akolekar, Chaffee & Howe, 1997; Zhao et al., 2004; Covarruvias et al., 2006). The characteristic bands observed in the metakaolinite were 1049, 806, 642, 571 and 428 cm-1, with three broad bands centred at 1049, 806 and 428 cm-1. A significant shift of the Si-O vibration bands at 1034 and 1012 cm-1 in kaolinite to a higher frequency band at 1049 cm-1 in metakaolinite was observed, which has been assigned to amorphous SiO2, as reported by some authors (Sinha et al., 1995; Valcke, Engels & Cremers, 1997; Qiu et al., 2004). The stretching vibration of Al (O,OH)6 octahedra in the kaolinite (Lambert, Minman & Fripiat, 1989) is observed at 539 cm-1, but is substituted by a peak at 571 cm-1 corresponding to the vibration band of AlO4 tetrahedron in metakaolinite; these peaks indicate the formation of the disordered metakaolinite phase.
The FT-IR spectra over the range 400-4000 cm-1 corresponding to the transformation of kaolinite and metakaolinite in NaF solutions are illustrated in Figure 4. The results obtained show that the starting materials showed no efficiency in relation to the reactivity under the experimental conditions to which they were subjected during their activation in the fluoride media, as demonstrated by the non-modification of the vibration bands characteristic of kaolinite and metakaolinite, which suffered no change in intensity or were displaced to other frequencies in contrast to what is reported in previous studies. The characteristic peaks of the pre-existing starting materials did not disappear, although they tend to weak with longer reaction times.
2.3. Scanning Electron Microscopy
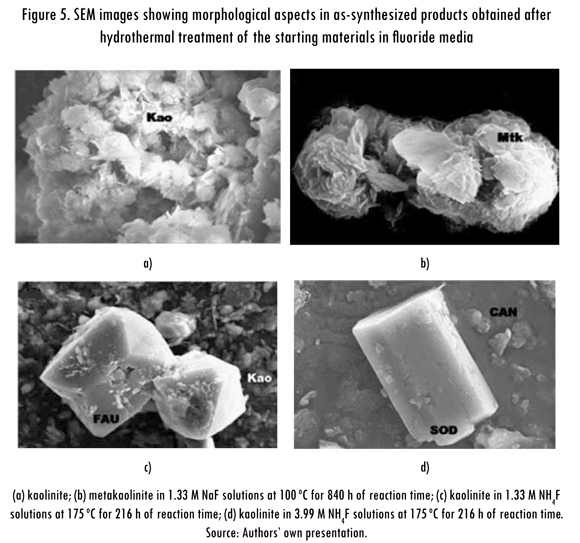
SEM micrographs of representative synthesis products obtained after hydrothermal reaction of the starting materials in fluoride media are illustrated in Figure 5. Only spheroidal and flower-like morphologies as well as the relict kaolinite (Figure 5a) or metakaolinite (Figure 5b) flaky crystals are observed, but not the characteristic morphologies of zeolites Na-P1, Linde Type Y and chabazite. Figure 5c shows spheroidal morphologies and relict flaky crystals of the starting material, as well as dodecahedral crystals of faujasite, displaying a typical cubic (octahedral) symmetry and simple twinning, from which flaky aggregates grow. As shown in Figure 5d, the transformation of kaolinite in NH.F solutions using SDA promoted the formation of sodalite and cancrinite. According to Kuperman et al. (1993), a fluoride-containing medium in the hydrothermal synthesis of zeolites favored the formation of larger zeolite crystals than those without the use of fluoride species.
2.4. Thermogravmetric Analysis
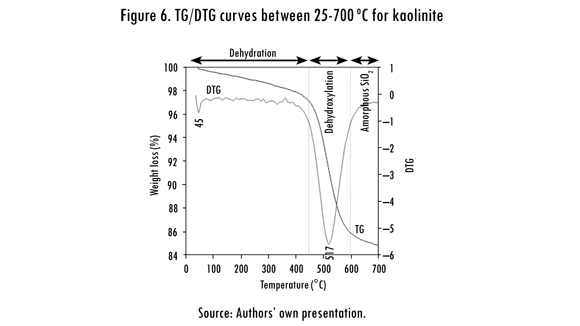
It is well known that the improvement of the properties of kaolinite by chemical methods is difficult due to its low reactivity (Ríos, 2008). Although the thermal transformation of kaolinite has been investigated for many years, a controversy about the process of dehydroxylation (elimination of the OH groups from the kaolinite structure) remains. The thermal decomposition of kaolinite is illustrated in the TGA (TG and DTG) curves on Figure 6 and can be summarized by a sequence of reactions. At < 100 oC, low temperature release of absorbed water in pores, on the surfaces, etc (water desorption), which depends on the nature of the kaolinite and the degree of disorder of stacking (Frost et al., 2003). Between ∼100-400 °C, a weight loss that can be correlated with a pre-dehydration process takes place as a result of the reorganization in the octahedral layer, first occurring at the OH of the surface (Balek & Murat, 1996). After dehydration, kaolinite goes through a pre-dehydroxylation state (Frost et al., 2003). According to Kakali et al. (2001), between ∼400-650 °C, the kaolinite dehydroxylation occurs, with the transformation to a non-crystalline phase, metakaolinite. On the other hand, Frost et al. (2003) considered that this process occurred between 450-550 oC.
The TGA (TG and DTG) curves for representative synthesis products are illustrated in Figure 7. These curves in the presence of fluoride ions exhibit several peaks, which could be generally correlated with different dehydration steps: 23-140, 140-220 and 400-600 °C. The position of these DTG peaks and the number of dehydration steps may be attributed to the different compensating cation-water binding energies, as well as to the different energy associated with the diffusion of the desorbed water through the porous structure of the zeolitic materials (Ríos, 2008). Their weight loss percentages reflect the water loss from the zeolite structure, and the amount of desorbed water is related with the number of compensation cations in the zeolite framework (Covarrubias et al., 2006). Notice that a significant mass loss occurs before 300 °C, which can be attributed to the moisture that is present in the mixtures.
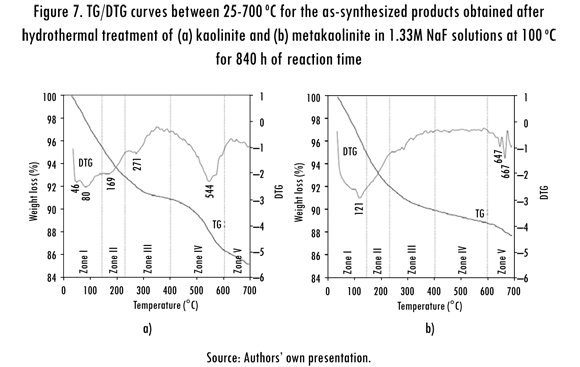
The thermograms of the mixtures containing traces of zeotypes reveal that the water of hydration evolved from all mineral phases. The peaks observed between 23-140° Care due to desorption of the occluded water in zeolitic materials (zone I). The presence of zeolitic water is revealed by the peak between 140220 oC (zone II). The peak in the zone III may be considered to be correlated with charge compensation between zeolite cations and fluoride ions, and incorporation of fluoride ions located in small cages within the zeolite framework as suggested by Camblor, Corma, and Valencia (1998). The framework containing Al species (zone IV). The dehydroxilation of the zeolitic material is registered between 600700 °C (zone V). However, it is dificult to distinguish between zeolite phases that belong to a complex mixture based on TGA data only.
3. Discussion
The reactivity of kaolinite and metakaolinite in fluoride media under hydrothermal conditions is discussed here and can be divided into a number of steps: dissolution of the starting materials in fluoride media releasing Si (preparation of the reaction gel) and hydrothermal reaction at several reaction temperature and time. It is suggested in this study that due to the affinity of silicon for fluoride, and the ability of silicon to expand its coordination number that the Si-F linkage should be formed. However, why was this mechanism non operable under the experimental conditions used in this study? The chemistry of evolution of the system is very complex due to several factors.
According to Zones et al. (2005), one of the most exciting developments in zeolite synthesis has been the discovery in recent years by researchers in Valencia (Spain), who have prepared very open-framework all-silica materials in highly concentrated fluoride reactions. Most conventional high silica zeolite syntheses would occur at H2O/SiO2 levels of 20 or greater and pH = 10-12, rather than the near-neutral HF conditions. It is very important to understand how H2O/SiO2 affects the product selectivity and what the behavior of fluoride anion might be in both this context and in the presence of different SDAs (Zones et al., 2005).
Does the silica serve as a reservoir from which to transport highly fluorinated silicate species to sites in solution (modest as the quantity may be) for nucleation in the presence of the SDA to occur? If this is a viable transport mechanism, it must operate at low concentrations because nucleation induction periods can be quite long in this fluoride medium. But surely if there is more than one species an equilibrium among them will be influenced by overall reaction concentration (Zones et al., 2005). The gel chemistry is completely different here, providing a further opportunity to prepare new zeolites, but the pH of fluoride-containing gels should be neutral-to-acidic, which was not obtained in the experiments (pH = 10.4-12.2) probably because the experimental conditions were not appropriated, as revealed by the occurrence of relitic phases of the raw materials along with a poorly crystallized mixture of several zeotypes.
The fact that the kaolinite and metakaolinite show a poor reactivity in fluoride media under the experimental conditions used in this study, explain why only traces of zeolites were obtained or did not grow, suggesting that the interaction between the ions in solution due to pH conditions is a key factor for inhibiting the synthesis process. Therefore, there were centres for the growth and anchorage of nuclei in zeolite formation. For a better understanding of the gel chemistry for the successful synthesis, additional experiments should be carried out under optimum reaction conditions, including the use of appropriate gel molar compositions and the ageing before the hydrothermal synthesis as key steps for the successful synthesis, taking into account that there are several controlling parameters in the success to nucleate and grow zeolite phases. They strongly affect either the nucleation and growth processes.
Conclusions
This work shows that the attempts to synthesise zeolitic materials from kaolinite and metakaolinite in fluorine media have so far not been successful, resulting in greater impurity and/or poorer crystallinity. It should be noted that different experimental conditions (time and temperature of reaction and SDA addition) on the formation process were tested, without being in a methodical search of the optimal experimental conditions. The pH of fluoride-containing gels obtained in the experiments (pH = 10.4-12.2) strongly affected the synthesis process. At higher mineralizer concentration, the addition of SDA and increasing the crystallization temperature promoted the formation of an amorphous product. Therefore, the results have been disappointing, in respect to those obtained by other authors. However, this preliminary study will certainly motivate further refinement of the synthesis method through advanced research.
References
AKOLEKAR, D.; CHAFFEE, A. & HOWE, R. F. The transformation of kaolin to low-silica X zeolite. Zeolites. 1997, vol. 19, num. 5, pp. 359-365. [ Links ]
ALKAN, M.; HOPA, C.; YILMAZ, Z. & GULER, H. The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous andMesoporous Materials. 2005, vol. 86, num. 1-3, pp. 176-184. [ Links ]
BACCOUCHE, A.; SRASRA, E. & MAAOUI, M.E. Preparation of Na-P1 and sodaliteocta-hydrate zeolites from interstratified illite-smectite. Applied Clay Science. 1998, vol. 13, num. 4, pp. 255-273. [ Links ]
BALEK, V. & MURAT, M. The emanation thermal analysis of kaolinite clay minerals. Thermo-chimica Acta. 1996, nums. 282-283, pp. 385-397. [ Links ]
BARRER, R.M. Hydrothermalchemistry of zeolites. 1st ed. London: Academic Press, 1982. [ Links ]
BARRER, R.M.; COLE, J.F. & STICHER, H. Chemistry of soil minerals. Part V Low temperature hydrotermal transformations of kaolinite. Journal of the Chemical Society A: Inorganic, Physical, Theoretical. 1968, pp. 2475-2485. [ Links ]
BARRETT, PA.; CAMBLOR, M.A.; CORMA, A.; JONES, R.H. & VILLAESCUSA, L.A. Synthesis and Structure of As-Prepared ITQ-4, A Large Pore Pure Silica Zeolite: The Role and Location of Fluoride Anions and Organic Cations. The Journal of Physical Chemistry B. 1998, vol. 102, num. 21, pp. 4147-4155. [ Links ]
BAUER, A. & BERGER, G. Kaolinite and smectite dissolution rate in high molar KOH solutions at 35 ° and 80 °C. Applied Geochemistry. 1998, vol. 13, num.7, pp. 905-916. [ Links ]
BAUER, A.; VELDE, B. & BERGER, G. Kaolinite transformation in high molar KOH solutions. Applied Geochemistry. 1998, vol. 13, núm. 5, pp. 619-629. [ Links ]
BOUKADIR, D.; BETTAHAR N. & DERRICHE, Z. Synthesis of zeolites 4A and HS from natural materials. Annales de Chimie Science des Matériaux. 2002, vol. 27, num. 4, pp. 1-13. [ Links ]
BRECK, D.W Zeolite molecular sieves: structure, chemistry and use. New York: John Wiley, 1974. [ Links ]
CAMA, J.; AYORA, C.; QUEROL, X. & GANOR, J. Dissolution kinetics of synthetic zeolite NaP1 and its implication to zeolite treatment of contaminated waters. Environmental Science and Technology. 2005, vol. 39, num. 13, pp. 4871-4877. [ Links ]
CAMBLOR, M.A.; CORMA, A. & VALENCIA, S. Synthesis in fluoride media and characterisation of aluminosilicate zeolite beta. Journal of Materials Chemistry. 1998, vol. 8, num. 9, pp. 2137-2145. [ Links ]
CAÑIZARES, P.; DURÁN, A.; DORADO, F. & CARMONA, M. The role of sodium mont-morillonite on bounded zeolite-type catalysts. Applied Clay Science. 2000, vol. 16, nums. 5-6, pp. 273-287. [ Links ]
CHANDRASEKHAR, S. & PRAMADA, P.N. Investigation on the synthesis of zeolite NaX from Kerala kaolin. Journal of Porous Materials. 1999, vol. 6, num. 4, pp. 283-297. [ Links ]
CHEZEAU, J.M.; DELMOTTE, L.; GUTH, J.L. & SOULARD, M. High-resolution solid-state 29Si and 13C n.m.r. on highly siliceous MFI-type zeolites synthesized in nonalkaline fluoride medium. Zeolites, 1989, vol. 9, núm. 1, pp. 78-80. [ Links ]
CHOROVER, J.; CHOI, S.; AMISTADI, M.K.; KARTHIKEYAN, K.G.; CROSSON, G. & MUELLER, K.T. Linking Cesium and Strontium uptake to kaolinite weathering in simulated tank waste leachate. Environmental Science Technology. 2003, vol. 37, núm. 10, pp. 2200-2208. [ Links ]
CORMA, A. Towards a rationalization of zeolite and zeolitic materials synthesis. Studies in Surface Science and Catalysis. 2004, vol. 154, num. 1, pp. 25-40. [ Links ]
COVARRUBIAS, C.; GARCÍA, R.; ARRIAGADA, R.; YANEZ, J. & GARLAND, T. Cr(III) exchange on zeolites obtained from kaolin and natural mordenite. Microporous and Meso-porous Materials. 2006, vol. 88, num. 1-3, pp. 220-231. [ Links ]
DAVIS, M.E. & ZONES, S.I. A perspective on zeolite synthesis: How do you know what you'll get? In: Synthesis of Porous Materials: Zeolites, Clays and Nanostructures, M.L. Occelli and H. Kessler (Eds.). New York: Marcel Dekker, 1997, pp. 1-34. [ Links ]
DEMORTIER, A.; GOBELTZ, N.; LELIEUR, J.P & DUHAYON, C. Infrared evidence for the formation of an intermediate compound during the synthesis of zeolite Na-A from metakaolin. International Journal of Inorganic Materials. 1999, vol. 1, num. 2, pp. 129-134. [ Links ]
EGEBLAD, K.; KUSTOVA, M.; KLITGAARD, S.K.; ZHU, K. & CHRISTENSEN, C.H. Mesoporous zeolite and zeotype single crystals synthesized in fluoride media. Microporous and Mesoporous Materials. 2007, vol. 101, num. 1-2, pp. 214-223. [ Links ]
FÉRONA, B.; GUTH, J.L. & MIMOUNI-ERDDALANEA, N. Influence of the presence of NaF on the crystallization of zeolite A (LTA): First evidence for the existence of fluo-rosodalite, the missing end-member of the halosodalite series. Zeolites. 1994, vol. 14, num. 3, pp. 177-181. [ Links ]
FLANIGEN, E.M. & PATTON, R.L. US 4 073 865 (1978). FROST, R.L.; HORVÁTH, E.; MAKÓ, E.; KRISTÓF, J. & RÉDEY A. Slow transformation of mechanically dehydroxylated kaolinite to kaolinite - an aged mechanochemically activated formamide - intercalated kaolinite study. ThermochimicaActa. 2003, vol. 408, nums. 1-2, pp. 103-113. [ Links ]
FROST, R.L.; MAKÓ, E.; KRISTÓF, J. & KLOPROGGE, J.T. Modification of kaolinite surfaces through mechanochemical treatment - a mid-IR and near-IR spectroscopic study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2002, vol. 58, num. 13, pp. 2849-2859. [ Links ]
HOCH, M. & BANDARA, A. Determination of the adsorption process of tributyltin (TBT) and monobutyltin (MBT) onto kaolinite surface using Fourier transform infrared (FTIR) spectroscopy. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2005, vol. 253, nums. 1-3, pp. 117-124. [ Links ]
HOUSSIN, Ch.J.Y. Nanoparticles in zeolite synthesis. PhD Thesis. Eindhoven, North Brabant: Eindhoven University of Technology, 2003. [ Links ]
JACOBS, PA. & MARTENS, J.A. Synthesis of high-silica aluminosilicate zeolites. New York: Elsevier Science, 1987. [ Links ]
JIANG, J.; JORDA, J.L.; YU, J.; BAUMES, L.A.; MUGNAIOLI, E.; DIAZ-CABANAS, M.J.; KOLB, U. & CORMA, A. Synthesis and structure determination of the hierarchical mesomicroporous zeolite ITQ-43. Science. 2011, vol. 333, num. 6046, pp. 1131-1134. [ Links ]
KAKALI, G.; PERRAKI, T.; TSIVILIS, S. & BADOGIANNIS, E. Thermal treatment of kaolin: the effect of mineralogy on the pozzolanic activity. Applied Clay Science. 2001, vol. 20, nums.1-2, pp.73-80. [ Links ]
KATO, M.; ITABASHI, K.; MATSUMOTO, A. & TSUTSUMI, K. Characteristics of MOR-framework zeolites synthesized in fluoride-containing media and related ordered distribution of Al atoms in the framework. The Journal of Physical Chemistry B. 2003, vol. 107, num. 8, pp. 1788-1797. [ Links ]
KIM, D.S.; CHANG, J-S.; HWANG, J-S.; PARK, S-E. & KIM, J.M. Synthesis of zeolite beta in fluoride media under microwave irradiation. Microporous and Mesoporous Materials. 2004, vol. 68, nums. 1-3, pp. 77-82. [ Links ]
KLIMKIEWICZ, R. & DRAG, E.B. Catalytic activity of carbonaceous deposits in zeolite from halloysite in alcohol conversions. Journal of Physics and Chemistry of Solids. 2004, vol. 65, num. 2-3, pp. 459-464. [ Links ]
KOLLER, H.; WOLKER, A.; VILLAESCUSA, L.A.; DÍAZ-CABAÑAS, M.J.; VALENCIA, S. & CAMBLOR, M.A. Five-coordinate silicon in high-silica zeolites. Journal of the American Chemical Society. 1999, vol. 121, num. 14, pp. 3368-3376. [ Links ]
KRISTÓF, J.; MINK, J.; HIRVATH, E. & GABOR, M. Intercalation study of clay minerals by Fourier transform infrared spectrometry. Vibrational Spectroscopy. 1993, vol. 5, num. 1, pp. 61-67. [ Links ]
KUBOTA, Y, HELMKAMP, M.M., ZONES, S.I. & DAVIS, M.E. Properties of organic cations that lead to the structure-direction of high silica molecular sieves. Microporous Materials. 1996, vol. 6, num. 4, pp. 213-229. [ Links ]
KUPERMAN, A.S.; OLIVER, S.; OZIN, G.A.; GARCES, J.M. & OLKEN, M.M. Non-aqueous synthesis of giant crystals of zeolites and molecular sieves. Nature. 1993, vol. 365, num. 6443, pp. 239-242. [ Links ]
LAMBERT, J.F.; MINMAN, W.S. & FRIPIAT, J.J. Revisiting kaolinite dehydroxylation: A silicon-29 and aluminum-27 MAS NMR study. Journal of the American Chemical Society. 1989, vol. 111, num. 10, pp. 3517-3522. [ Links ]
LIN, D.-Ch.; XU, X.-W; ZUO, F. & LONG, Y-C.Crystallization of JBW, CAN, SOD and ABW type zeolite from transformation of meta-kaolin. Microporous and Mesoporous Materials. 2004, vol. 70, nums. 1-3, pp. 63-70. [ Links ]
LIU, Q.; SPEARS, D.A. & LIU, Q. MAS NMR study of surface-modified calcined kaolin. Applied Clay Science. 2001, vol. 19, num. 1-6, pp. 89-94. [ Links ]
LOBO, R.F., ZONES, S.I. & DAVIS, M.E. Structure-direction in zeolite synthesis. Journal of Inclusion Phenomena and Macrocyclic Chemistry. 1995, vol. 21, nums. 1-4, pp. 47-78. [ Links ]
LUSSIER, R. A novel clay-based catalytic material-preparation and properties. Journal of Catalysis. 1991, vol. 129, num. 1, pp. 225-237. [ Links ]
MACKENZIE, R.C. Differential thermal analysis. Vol. 1. London: Academic Press, 1970. [ Links ]
MURAT, M.; AMORKRANE, A.; BASTIDE, J.P. & MONTANARO, L. Synthesis of zeolites from thermally activated kaolinite.Some observations on nucleation and growth. Clay Minerals. 1992, vol. 27, num. 1, pp. 119-130. [ Links ]
PERISSINOTTO, M.; STORARO, L.; LENARDA, M. & GANZERLA, R.J. Solid acid catalysts from clays: Acid leached metakaolin as isopropanol. Journal of Molecular Catalysis A: Chemical. 1997, vol. 121, num. 1, pp. 1103-109. [ Links ]
QIU, G.; JIANG, T.; LI, G.; FAN, X. & HUANG, Z. Activation and removal of silicon in kaolinite by thermochemical process. Scandinavian Journal of Metallurgy. 2004, vol. 33, num. 2, pp. 121-128. [ Links ]
REES, L.VC. & CHANDRASEKHAR, S. Hydrothermal reaction of kaolinite in presence of fluoride ions at pH less than 10. Zeolites. 1993, vol. 13, num.1, pp. 534-541. [ Links ]
RÍOS, C.A. Synthesis of zeolites from geological materials and industrial wastes for potential application in environmental problems. PhD Thesis. Wolverhampton, West Midlands: University of Wolverhampton, 2008. [ Links ]
RÍOS, C.A.; WILIAMS, C.D. & FULLEN, M.A. Nucleation and growth history of zeolite LTA synthesized from kaolinite by two different methods. Applied Clay Science. 2009, vol. 42, nums. 3-4, pp. 446-454. [ Links ]
SAIKIA, N.J.; BHARALI, D.J.; SENGUPTA, P.; BORDOLOI, D.; GOSWAMEE, R.L.; SAIKIA, P.C. & BOTHAKUR, P.C. Characterization, beneficiation and utilization of a kaolinite clay from Assam, India. Applied Clay Science. 2003, vol. 24, nums. 1-2, pp. 93-103. [ Links ]
SCHMIDT-WINKEL, P; YANG, P; MARGOLESE, D.I.; CHMELKA, B.F. & STUCKY G.D. Fluoride-induced hierarchical ordering of mesoporous silica in aqueous acid-syntheses. Advanced Materials. 1999, vol. 11, num. 4, pp. 303-307. [ Links ]
SERRANO, D.P; VAN GRIEKEN, R.; SANCHEZ, P; SANZ, R. & RODRIGUEZ, L. Crystallization mechanism of all-silica zeolite beta in fluoride medium. Microporous and Mesoporous Materials. 2001, vol. 46, num. 1, pp. 35-46. [ Links ]
SINHA, PK.; PANIKER, P.K. & AMALRAJ, R.V Treatment of radioactive liquid waste containing caesium by indigenously available synthetic zeolites: A comparative study. Waste Management. 1995, vol. 15, num. 2, pp. 149-157. [ Links ]
SZOZTAK, R. Handbook of molecular sieves. London: Blackie Academic and Professional, 1998. [ Links ]
TAVOLARO, A.; MOSTOWICZ, R.; CREAA, F.; NASTRO, A.; AIELLOA, R. & NAGY, J.B. Formation of MFI crystalline zeosilites from fluoride-containing silicate gels. Zeolites. 1992, vol. 12, num. 6, pp. 756-761. [ Links ]
VALCKE, E.; ENGELS, B. & CREMERS, A. The use of zeolites as amendments in radiocae-sium- and radiostrontium-contaminated soils: A soil-chemical approach. Part II: Sr-Ca exchange in clinoptilolite, mordenite and zeolite A. Zeolites. 1997, vol. 18, nums. 2-3, pp. 212-217. [ Links ]
VAN DE MAREL, H.W & BEUTELSPACHER, H. Atlas of infrared spectroscopy of clay minerals and their admixtures. Amsterdam: Elsevier, 1976. [ Links ]
XU, M.; CHENG, M.; BAO, X.; LIU, X. & TANG, D. Growth of zeolite KSO1 on calcined kaolin microspheres.Journal of Materials Chemistry. 1999, vol. 9, num. 12, pp. 2965-2966. [ Links ]
ZHAO, H.; DENG, Y; HARSH, J.B.; FLURY M. & BOYLE, J.S. Alteration of kaolinite to cancrinite and sodalite by simulated hanford tank waste and its impact on cesium retention. Clays and Clay Minerals. 2004, vol. 52, num. 1, pp. 1-13. [ Links ]
ZONES, S.I.; DARTON, R.J.; MORRIS, R. & HWANG, S.-J. Studies on the role of fluoride ion vs. reaction concentration in zeolite synthesis. Journal of Physical Chemistry B. 2005, vol. 109, num. 1, pp. 652-661. [ Links ]













