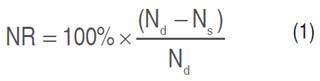Cocoa (Theobroma cacao L.) is a species from the Theobroma genus and the Malvaceae family. This species is the most widely cultivated plant as it has high economic value (Najihah et al., 2018). Cocoa beans are the raw material for chocolate and cosmetics. The average cocoa production that comes from smallholder plantations from 2011 - 2018 was 649,807 t annually or around 94.32% of total national production (Ruslan and Prasetyo, 2021). In Indonesia, cocoa cultivation is conducted using agroforestry and full-sun systems (Witjaksono, 2016). Sowing protective plants to reduce direct sunlight exposure could increase cocoa production (Wartenberg et al., 2019). High temperatures have negative impacts on cocoa plants, such as eco-physiological stress, environmental changes, and decreased CO2 assimilation. Shade plants play an important role to enhance cocoa plants' physiology and the quality of soil nutrients. The benefits of cacao cultivation under coconut trees are that it can increase land use efficiency, sunlight, and control Helopeltis as the main pest of cocoa (Adam et al., 2017). The cocoa-coconut intercropping system does not create competition for sunlight because cocoa is a plant that can grow in 40-80% shade (Latha et al. 2017).
Leaves are among the vital organs of a plant, which can affect plant growth and are one of the primary organs in the assimilation process that affects plant production. Furthermore, leaves are also correlated with the physiological functions of the plants, such as specific leaf area (SLA), photosynthetic capacity, nitrogen and phosphorus content, respiration rate, and leaf age (Wright et al., 2004). SLA is the leaf area per dry mass that is used as a parameter to assess the plant's carbon content and water status (Ali et al., 2017). SLA can indicate the relative growth rate of plants. Also, changes in SLA can represent the structure of leaves and their nutrient content because SLA is mostly affected by photosynthesis activity (Gong and Gao, 2019).
Macroelements, such as nitrogen, phosphorus, potassium, and calcium, play an essential role in several physiological processes (Ashraf et al., 2014). Resorption is a process to use nutrients efficiently by transferring nutrients from senescence leaves to young leaves (Housman et al., 2012). Environmental factors, such as the dry season with low water supply, affect the resorption process. The senescence leaves use 50% of their N and P components, enzymes, lecithin, and nucleic acids to be translocated to seeds, roots, young leaves, and other parts of plants via the phloem tissue (Tang et al., 2013;). There were indications of NPK limitation in all cropping patterns used because the NPK concentration in cocoa was lower than the ideal level without fertilization. C. indicum tree planting system at a distance of 8 m×16 m and G. sepium at a distance of 12×12 m was preferred over C. indicum at a distance of 8×8 m for T. cacao. This was because the high-density distance can lead to the depletion of organic matter. The genotype of cocoa affects the response or interaction with the cultivation environment, and will subsequently also affect the efficiency of nutrients (Rosas-Patiño et al., 2019).
Plants adapt to changing water content by metabolic adaptation to deal with osmotic pressure and trigger the synthesis of the osmoprotectants, which are proline, protein, and sugar. Those osmoprotectants regulate osmotic potentials and protect the cells from drought damage and regulate drought stress (Li et al., 2015). Proline is the main organic osmolyte compound during abiotic stresses. Furthermore, proline plays a critical role in the osmotic regulating compound and plant structure protection. Proline accumulation is more extensive during the day than at night to protect plants from UV radiation in Barley (Fedina et al., 2002). The adjustment of osmotic compounds can be evaluated by the accumulation of sugars to balance water potential during drought stress in cotton (Jamal et al., 2015). The more plants experience water shortages, the proline level increases as a survival effort of the plant. According to Janani et al. (2019), cocoa clones that were subjected to drought stress and produced the highest proline content also showed high-stress tolerance, thus potentially producing cocoa.
The influence of seasons and planting patterns on cocoa growth is needed to discover the differences in physiological responses. The present study aimed to analyze differences in SLA, N, and P resorption in leaves, and proline and glucose concentration in cocoa roots in different seasons and planting patterns.
MATERIALS AND METHODS
Experimental site and design
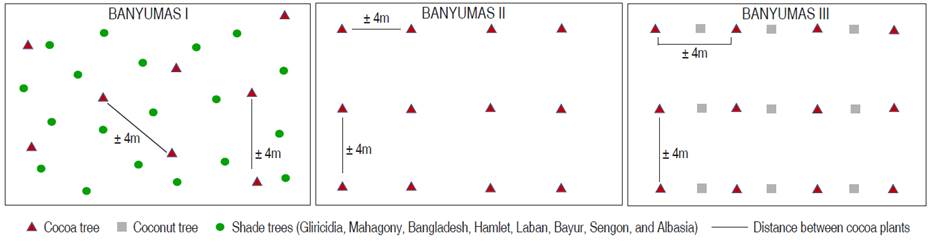
The present study was conducted in 2015 and 2016 in Plana village, Somagede, Banyumas, Central Java, Indonesia (Type climate A based on Kuppen climate classification). Based on information from Sugito et al. (2019), Plana village has an altitude of 300 m, so it is classified as medium land. The sampling areas were determined by the planting patterns of the cocoa plantation (Figure 1). This land is rainfed soil and NPK fertilizer (10 kg plant-1) is only applied once a year in the early rainy season from month 1 to month 2. Leaf trimming of young shoots and twigs that had many leaves according to Angela and Efendi (2015) was carried out every 2 months depending on the vegetation's density. For maintenance pruning, removed branches had a diameter of < 2.5 cm, heavy pruning was also carried out when the plant was too dense. This pruning also aimed to stimulate the growth of flowers and fruit. Insecticides were not used in this study because of the absence of insect pests on the observed cocoa plants (cocoa hybrid clone). In areas 2 and 3, the number of cocoa plants in each area was 12 trees. The age of cocoa, coconut, and other plants are 8 years, 10 years, and about 10 years, respectively. The percentage of shade plants in area 1 was 75%. Each area was about 2000 m2 and the planting distance between cocoa plants was 3 m (Figure 1). A completely randomized design with 12 replications was used in this study.
From 24 cocoa plants, 12 leaves per area were picked up in each area to evaluate the SLA and N and P content. The analyses on the cocoa tree roots were conducted to examine the proline and glucose content. Proline and glucose were quantified in three periods (6-7 June 2015, 31 October 2015, and 26-27 March 2016) to show a complete seasonal profile in Indonesia. According to BPS (2017), Banyumas has the highest rainfall of 113.25 mm3 in June (rainy season) and the lowest of 0 mm3 (dry season) in October 2015. The rainfall took place in all months observed in 2016 with 302 mm3 of rainfall in March 2016.
Environmental and weather factors determination
The microclimate was measured from January 2015 to December 2016 every week. Air temperature, humidity, pH, temperature, and groundwater content were measured in the present study. Air temperature and humidity were measured by using a thermohygrometer. pH, temperature, and groundwater content were measured using a tensiometer from 12.00 a.m. to 02.00 p.m.
Specific leaf area (SLA) analysis
SLA is the ratio of leaf area to dry weight (cm2 g-1). SLA observations were conducted using adult cocoa leaves with a similar leaf index color according to Roderick et al. (1999). Leaf samples (12 leaves area-1) were obtained from plant branches, wrapped in aluminum foils, and stored in a cool box to keep them fresh. The leaf area (cm2) was measured using millimeter block paper. The leaves were dried using an oven to calculate the dry weight (cm2 g-1). SLA evaluation was conducted with three different vegetation areas in 2015 and 2016.
Analysis of nitrogen (N) and phosphorus (P)
N and P content analysis was conducted in adult and senescence leaves of cocoa plants on 26-31 January 2015 and 3-9 October 2016. The N leaf content was evaluated using the Kjeldahl method (Singh et al., 2015). P content of the leaf was evaluated using the Morgan-Wolf method as a sanitizer based on Jeshni et al. (2017). N and P resorption measurement was conducted according to Li et al. (2019a) by Equation 1.
Where, NR: nitrogen resorption (%); Nd: adult leaf nitrogen (%); Ns: senescence leaf nitrogen (%)
Proline analysis
Fine root samples were obtained using a soil core at 20 cm depth in midday (12.00-14.00) on 6-7 June 2015 (early dry season), 31 October 2015 (late dry season), and 26-27 March 2016 (late rainy season). Proline accumulation was analyzed based on Bates et al., (1973) method. A total of 0.5 g of dry root was homogenized with 10 mL of 3% sulfosalicylic acid for 72 h and then filtered. About 2 mL of filtrate was reacted with 2 mL of ninhydrin acid and glacial acetic acid. The samples were heated at 100 °C for 1 h. The filtrate was then placed into a glass cup with ice. A mixture of filtrate, ninhydrin, and glacial acetic acid was added to toluene and stirred for 15-20 s. The obtained red toluene with proline was measured using a spectrophotometer at 520 nm wavelength and compared to a standard proline curve to obtain the proline level of the sample.
Glucose analysis
Phenol method with glucose solution was used to determine the root's glucose content according to Chow and Landhäusser (2004). The reagents were 5% phenol solution in water, 95.5% H2SO4 with a density of 1.84, and a standard glucose solution. The used equipment was a spectrophotometer and a 25 °C water bath. The standard curve was made with 2 mL of a standard glucose solution with 0, 10, 20, 30, 40, and 60 μL glucose. The fine root samples were obtained similarly to proline analysis. The obtained samples were immediately heated at 60 °C for 60 s. Then, it was added with 1 mL of 5% phenol solution and mixed. After that, it was added with 5 mL of the sulfuric acid solution for 10 min, constant shaking, and placed in a water bath for 15 min. The absorbance was measured at 490 nm for hexose and 480 nm for pentose and uronic acid.
RESULTS AND DISCUSSION
Climatology characteristics
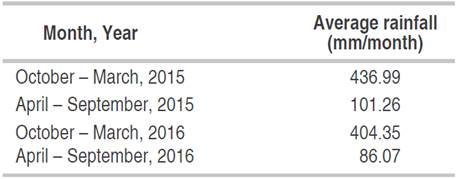
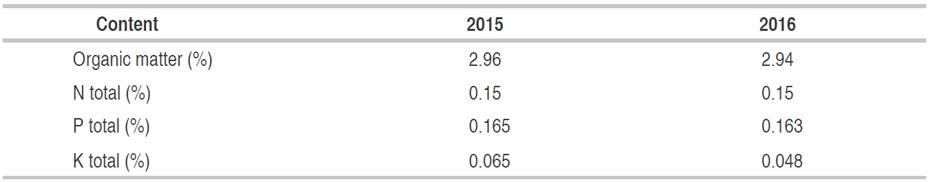
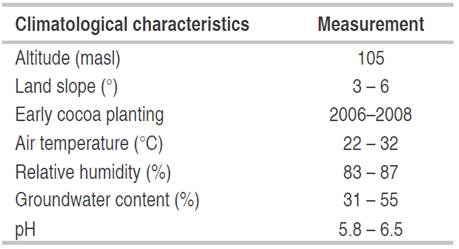
The climatology characteristics of the plantations' locations was shown in Table 1. The annual weekly or monthly rainfall distribution is more important than the total amount during the year because cocoa plants are more suitable in the months with not too high rainfall. Cocoa plantations generally have around 1250 to 3000-mm of rainfall every year. Based on the rainfall data of the location, the rainfall in plantations has the appropriate amount for cocoa plants' growth (Table 2). For optimal growth of cocoa plants, a maximum temperature between 30-32 °C, a minimum temperature between 18-21 °C (Sitohang and Siahaan, 2018), and relative humidity of 100% at night and between 70-80% during the day are needed conditions. Table 3 shows the organic matter, nitrogen (N), phosphate (P), and potassium (K) of the Banyumas soil (sampling site). Nitrogen, phosphorus, and potassium were 0.13-10.16%, 0.155-0.172%, and 0.03-0.1%, respectively (Table 3). Generally, the observed mineral content indicated that the soil is fertile at the sampling sites.
Table 1 Location and climatological characteristics of cocoa plantations in Plana Village, Banyumas, Central Java.
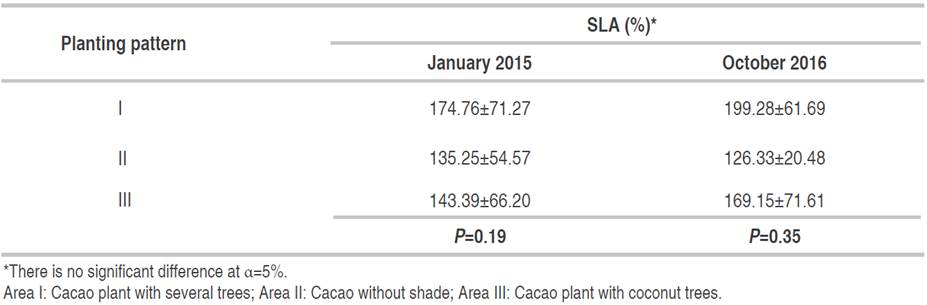
Specific leaf area
There was no significant difference between planting patterns for specific leaf area (SLA) in January 2015. Furthermore, there was a non-significant difference in the three planting patterns in October 2016 (Table 4). At the peak of the dry season in October 2016, the SLA in plot II decreased of 8.92%, while in plots I and III increased of 24.52% and 25.76%, respectively compared to January 2015. However, the sampling was done during a relatively high rainfall period in January 2015 and a dry period in October 2016.
SLA is a morphological parameter of nutrients a moisture availability that can be influenced by environmental conditions and the age of the leaf. SLA plays an important role in determining plant productivity, changes in leaf SLA indicate changes in leaf structure and nutrient content. In addition, differences in leaf SLA are influenced by soil and environmental climatic factors (Gong and Gao, 2019). Low SLA is due to the leaf's adaptation to survive, store nutrients, and avoid drought. The small size helps to regulate the temperature and water use efficiency for photosynthesis in dry and low water supply conditions (Karavin, 2013).
The increase in SLA is due to the increase in the number of cocoa leaves and may be due to a decrease in light intensity (Zang et al., 2016). Low SLA values do not reflect or indicate low photosynthetic activity and vice versa. However, in a water shortage condition, the temperature around the surface of the cocoa unshaded is higher than those in the shade, which causes faster groundwater evaporation, resulting in a reduced photosynthesis rate for survival (Lahive et al., 2019). According to Xu et al. (2008), decreasing soil moisture can reduce leaf size, and eventually affect the rate of photosynthesis. Photosynthetic rate is associated with SLA; by increasing the light capture area per mass, high SLA improves photosynthesis (Goorman et al., 2011). Photosynthetic rate is associated with SLA; by increasing the light capture area per mass, a larger leaf size, and higher photosynthesis (Huang et al., 2021).
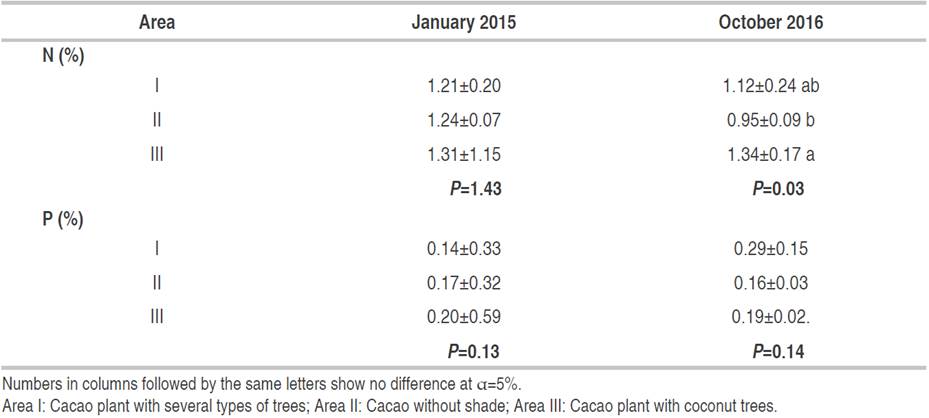
Resorption of nitrogen (N) and phosphorus (P)
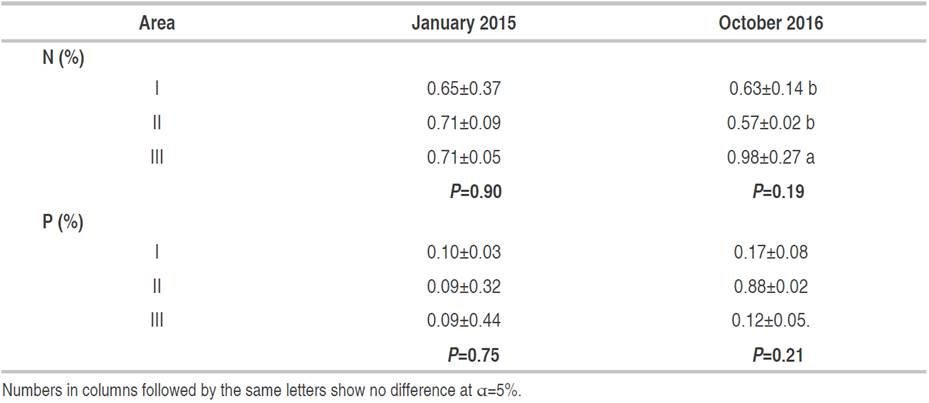
Table 5 shows the N and P contents of cocoa leaves. The average N content was higher in 2015 than in 2016 in areas I and II. As shown in Table 5, the N content was increased in area III during 2015 as well. The increased N content is needed by cocoa plants that grow with coconut plants. The P content was not significantly different from N content; the P content was lower in 2015 than in 2016 in three different vegetation areas. The highest P increase was found in area II at 877.78%. Moreover, the N and P content of cocoa leaves decreased in the senescence phase (Table 6).
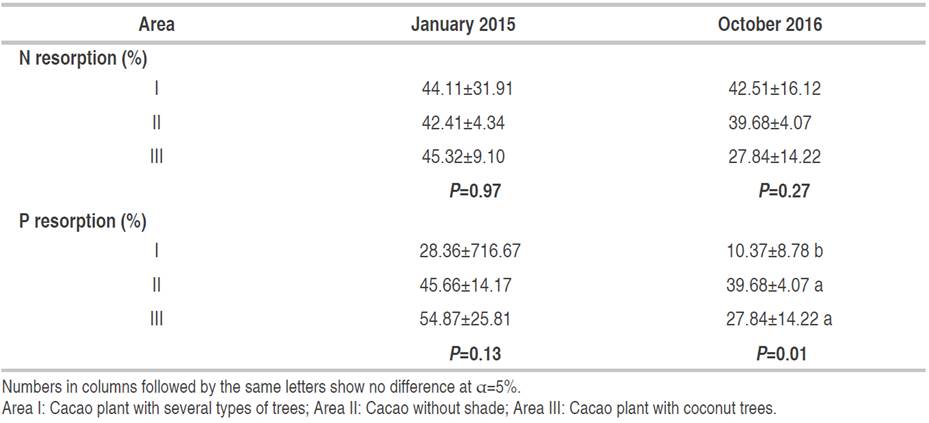
N and P resorption of cocoa leaves was higher in 2015 than in 2016 because of the different seasons (Table 7). The rainfall in Banyumas was low (almost 0 mm3) in October 2015 because of still early for the rainy season. The reduction in areas I-III was 3.63, 6.44, and 38.57%, respectively. Furthermore, the N resorption in area I was not significantly decreased because of the shade plant presence. In 2016, the decrease of P resorption in areas I-III was 63.43, 13.10, and 49.26%, respectively. This shows that the most significant decrease in P resorption was in area I and the lowest P resorption was in October (P=0.01) (Table 8).
Nitrogen plays a vital role in the growth process, leaf production, and protein content in plants. The dry season affects the absorption and transportation of N. It may reduce the transpiration and the permeability of the membrane. Besides, nutrient transfer from senescence leaves to underground organs increases nutrient use (Yang et al., 2016).
P absorption from the soil decreases in the dry season because of drought stress, reducing nutrient diffusion, and mass flow in grasses in the soil (Bista et al., 2018). The resorption ability depends on the nutrients in the soil and the environmental conditions, such as temperature and light. According to some authors, plants utilize about 5-80% N and 0-95% P through leaves (Aerts and Chapin, 2000). Furthermore, N resorption for each plant species ranges from 16 to 42%. Additionally, microclimate under the canopy increases the decomposition of N content (Sari et al., 2022). Area II had a good absorption of N. The root system of mature cocoa plants is stronger than the perennial or annual plant.
The most significant decrease in P resorption occurred in cocoa plants with coconut plants. Coconut trees have a root system up to 2 m wide and 0-60 cm below the soil surface making it easier to absorb nutrients than cocoa (Adam et al., 2017). Generally, the ability of plants to do P resorption is at 0 until 90%. It increases when the availability of P in the soil decreases (Mayor et al., 2014). The low rate of resorption is influenced by competition with other trees. In another study, N and P resorption were higher in plants affected by drought stress (Lobo-do-Vale et al., 2018). On the contrary, the P resorption tends to decrease in the present study.
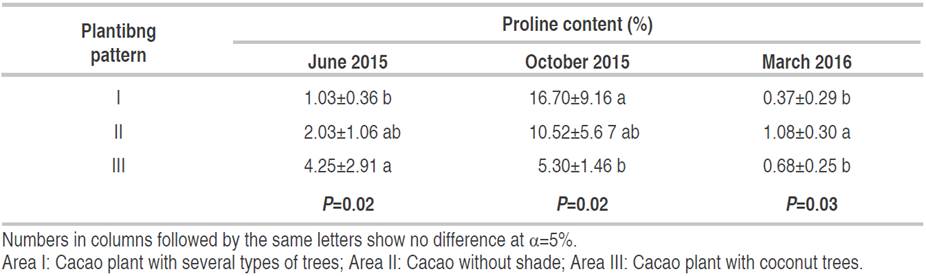
Proline content of cocoa root
The proline content in the cocoa plant root was increased in all planting patterns from June to October 2015 (Table 8) due to the drought. Based on the planting pattern, the proline content was significantly different (P=0.02) in June 2015, the difference between area I and area III was significant, 1.03% and 4.25%, respectively. In Oct 2015, the proline content in area I was significant (P=0.02) different and lower than area III, 16.7% and 5.30%, respectively. And in March 2016, the proline content in area II (1.08%) was significantly (P=0.03) higher than area I (0.37%) and Area III (0.68%).
Proline accumulation was relatively higher in cocoa plants root without shade trees compared to those with cocoa plants root in the area with coconut trees (Table 8). The increase in proline is due to drought stress during the dry season (Devaranavadagi et al., 2002). Proline is a substrate for respiration, a source of nitrogen, and other metabolisms during the dry season that was reported in wheat. The accumulation of proline during drought stress does not inhibit the biochemical reactions, but it acts as an osmoprotectant in corn (Molazem et al., 2010). Osmotic regulation triggers cell development and plant growth in the dry season in wheat (Keyvan, 2010). Osmotic adaptation maintains the cell's turgor to enlarge, influences plant growth and allows stomata to open to assimilating CO2 in Festuca (Man et al., 2011). The highest decrease in proline was 97.78% in area I from October 2015 to March 2016. The plants in areas with diverse vegetation have a small amount of proline because of the low transpiration process. It was because plants do not need to make osmotic adjustments like when drought stress increases proline (Robakowski et al., 2020).
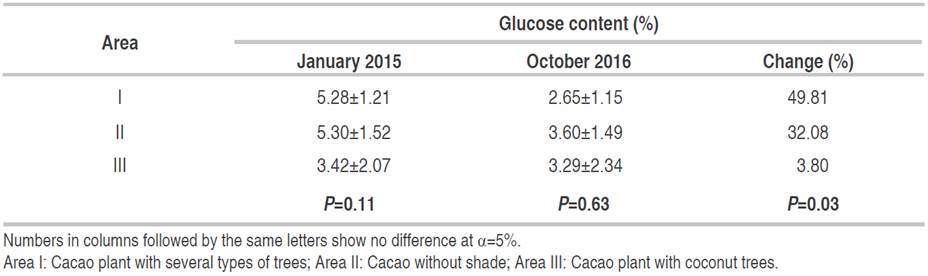
Glucose content of cocoa root
There were no significant differences in glucose content between planting patterns during 2015 and 2016 (Table 9).
The decrease in glucose levels in area I-III was 49.81%, 32.07%, and 3.8%, respectively, showing that the highest decrease in glucose content was in area I.
Plants have an osmotic adjustment mechanism for leaves and root turgor for growth, water absorption, cytokinin synthesis, and photosynthesis in black willow (Carpenter et al., 2008). High carbohydrate storage in leaves during the dry season can be used for the respiration process of Aleppo pine (Klein et al., 2011). Besides, higher amounts of sugar during the dry season are due to the translocation of carbohydrates from senescing leaves and the increased rate of photosynthesis at the beginning of the dry season. Sugars affect the growth, development, and metabolism of leaves, shoots, roots, and other plant organs (Ciereszko, 2018). According to Tezara et al. (2020), each clone of cocoa has a different physiological response to drought stress. Leaf N content, chlorophyll, and photochemical activity are reduced during drought, and the metabolism is disrupted. However, certain clones managed to survive in an environment with low water availability. Research by Rosas-Patiño et al. (2019) revealed that liming and fertilization affect nutrient efficiency and yields, in addition, the genotype of the cocoa and climate conditions also have an effect.
CONCLUSION
In conclusion, cocoa had different responses to cocoa root proline levels, leaf glucose levels, SLA, and leaf N and P resorption to deal with seasonal changes. Further study to correlate yields with proline or glucose concentrations is needed to determine whether sowing cocoa plants with other types of plants can be used to help farmers and stakeholders in managing cocoa cultivation to increase yields in efficient and sustainable ways.