INTRODUCTION
Passiflora edulis f. edulis, commonly known as purple passion fruit, is cultivated in many subtropical and tropical regions 1,500 m a.s.l. (Joy and Sherin, 2016), and especially in Colombia in altitudes between 1,600 and 2,300 m (Fischer et al., 2022), is one of the most sought-after exotic fruits in the global market, primarily due to its organoleptic properties, nutritional characteristics (macro and micronutrients), and the accumulation of secondary compounds with medicinal potential (Corrêa et al., 2016; He et al., 2020; Lozano-Montaña et al., 2021; Fonseca et al., 2022). Besides its culinary uses, there is emerging pharmaceutical interest, as recent studies have demonstrated its antioxidant, anti-inflammatory, antidiabetic, anxiolytic, anticancer, and antihypertensive properties (Carmona-Hernández et al., 2019, Alves et al., 2021, Hettiarachchi et al., 2022). In Colombia, the national production is increasing and is related to a higher percentage of exports every year. In the last years the production was of 24,798 t (Rodríguez-Polanco et al., 2022). However, in Colombia the diseases are serious problem for passion fruit plants production, causing losses in quantity and quality of the fruit, which can be very high when there are environmental conditions that favor the development of pathogens, such as higher altitudes and rainfall, allow the increase of the bacterium inoculum, which can spread from the leaves to the stems and fruits (Benítez and Hoyos, 2009; Rodríguez-Polanco et al., 2022).
Many factors contributing to the reduction in longevity and productivity in purple passion fruit plants, especially due to diseases caused by viral (Sepúlveda et al., 2022), bacterial or fungal etiologies (Joy and Sherin, 2016). Among these microorganisms Xanthomonas bacteria and Fusarium fungus are frequently involved (Fernández Barbosa and Suarez Meza, 2009; Benítez et al. 2011). Additionally, prolonged cultivation, the use of antibiotics, and poor agricultural practices have led to the development of resistance in some of these phytopathogens (Farfán et al., 2014).
The Xanthomonas genus, is a gram-negative bacteria and near 27 species are pathogenic to several plants including cane, beans, cabbage, banana, citrus, tomatoes, pepper, and rice between others and the genus is responsible for many crop diseases, causing economic losses worldwide (Nakayinga et al., 2021). Distinct genomic groups have been defined with X. axonopodis with many pathovars causing diseases on different host plants of agronomic significance. The symptoms include angular leaf lesions, blight, and wilt, stem exudates and stem canker. Other species is X. perforans pathogen that colonizes different growth stages of several plants in greenhouse or fields and causal agent of bacterial spot of tomato (Abrahamian et al., 2021).
Currently, host resistance is the most effective way to control this disease (Mansfield et al., 2012). Other methods include uprooting, burying and burning of infected plant tissues, sterilization of garden tools, and application of pesticides or antibiotics such streptomycin and copper-based compounds; however, resistance is widespread (Abrahamian et al., 2021).
In the case of the fungus, Fusarium oxysporum is one of the most destructive pathogens in agriculture, causing vascular disease in economically important crops (Abrahamian et al., 2021). The application of synthetic pesticides is the primary strategy to control disease. Nevertheless, fungicides have benefited crop production for decades, currently the use of such chemicals is restricted associated to overapplication has raised impact on the environment, contamination of drinking water, imbalance in the ecosystem, cause neurological effects in humans, severe lung injuries and the development of resistance is other of the principal problems (Seepe et al., 2021).
The foregoing motivates the search for new molecules as alternatives for the control of phytopathogens. The snake venoms have demonstrated antimicrobial activity over different microorganism including bacteria and fungus. These compounds include peptides and proteins with enzymatic function and the studies suggest that the components show diverse antimicrobial efficacy depending on the component and classes of microorganisms (Charvat et al., 2018). For instance, a PLA2 phospholipase obtained from the venom of Crotalus adamanteus demonstrated antimicrobial activity in vitro against S. aureus and Enterobacter aerogenes (Samy et al., 2014). Also, a lectin obtained from Bothrops leucurus was effective against Staphylococcus aureus and Enterococcus faecalis (Nunes et al., 2011). Likewise, a peptide derived from crotamine from Crotalus durissus terrificus (South American rattlesnake) induced marked antimicrobial activity (Falcao and Radis-Baptista, 2020).
Synthetic cationic peptides derived from a PLA2, from Bothrops asper, such as pEM-2 have shown activity against bacteria and fungus (Santamaría et al., 2005; Murillo et al., 2007). Part of the sequence of that same peptide allowed the construction of a new one called V3, which increased its activity and improved its therapeutic index (Memariani et al., 2017). A PLA2 with activity against S. aureus was identified from the venom of Porthidium nasutum from Antioquia-Colombia (Vargas et al., 2012). Likewise, from elapids of the genus Micrurus, a component was isolated that showed antibacterial activity on ATCC strain (Rey-Suárez et al., 2018). In the same way, venom Cathelicidins have demonstrated in vitro and in vivo antimicrobial activity (de Barros et al., 2019).
The aim of this work was to evaluate the antimicrobial properties of Bothrops asper and Porthidium nasutum venoms against phytopathogenic fungi and bacteria affecting Passiflora edulis f. edulis, given the need for alternatives to control of diseases in crops caused by these microorganisms.
MATERIALS AND METHODS
Venoms
Venom was obtained by manual extraction of Bothrops asper and Porthidium nasutum specimens collected in Antioquia and Choco, Colombia; and maintained in captivity at the serpentarium of Universidad de Antioquia (Medellin, Colombia). Venoms were centrifuged at 800 g for 15 min, and supernatants were lyophilized and stored at -20°C until use.
Isolation and identification of the microorganism
Samples of leaves from purple passion fruit (Passiflora edulis f. edulis) crops exhibiting disease symptoms caused by Xanthomonas or Fusarium were collected for the isolation of these microorganisms (Fig. 1).

Photos by Rey-Torres A., from a purple passion fruit crop in Colombia.
Figure 1. Typical alterations caused by phytopathogens in purple passion fruit. A) damage caused by Fusarium, showing plant chlorosis, growth retardation, wilting, fruit darkening, and dry stem. B) Bacteriosis caused by Xanthomonas, depicting leaves and fruits with necrotic spots and oily exudate.
For isolation, the diseased tissue was washed, and dried and several segments were cut in small pieces (0.5 cm) and placed on the surface of a potato dextrose agar PDA (Becton-Dickinson, USA) plate for Fusarium or nutrient agar (Becton-Dickinson, USA) plate for Xanthomonas. To obtain pure cultures, tips from development colonies were transferred to fresh PDA media or chocolate agar PolyViteX (BioMérieux, Brazil) for Xanthomonas. When a pure culture was observed, the identification of the microorganism was performed in the laboratory of Collection of Microorganism, Microbiology School, Universidad de Antioquia (CM-EM-UDEA)-Unique National Registry of Biological collections (RNC) No 250. For them, different strategies were used: direct with blue of lactophenol, gram stain; oxidase and catalase test. Finally, MALDI-TOF (MALDI-Biotyper) was employed to identify each species, as recommended by manufacturer.
Antimicrobial assay
Antimicrobial susceptibility testing was performed as described by Vargas et al. (2012), with some modifications. For the agar diffusion assay, Fusarium or Xanthomonas grown on PDA or Chocolate agar were suspended in sterile saline solution, turbidity was measured at 600 nm and adjusted to McFarland patron (absorbance of 0.5), then were seeded on agar on Mueller-Hinton agar plates (MERCK Darmstadt, Germany). After 10 µL of each P. nasutum or B. asper venom was added to bacterial agar media and incubated for 24 h to Xanthomonas or 48 h to Fusarium at room temperature (RT). A blank with sterile saline solution instead of venom served as negative control and antibiotic-antimycotic solution (SIGMA) served as a positive control or reference. Diameters of the bacterial growth inhibition zones were measured using a ruler. Each assay was performed in duplicate.
Minimum inhibitory concentration (MIC) by broth microdilution susceptibility test
The assay was performed as described by Vargas et al. (2012). Serial dilutions of each venom (500-0.97 µg mL-1) were prepared in 96-well microliter plates at a final volume of 50 µL saline solution; then, 100 µL/well of Mueller-Hinton broth, then 50 µL of the bacterial inoculum adjusted to McFarland patron (absorbance of 0.5) were added to each well and incubated at RT for 24 h. Inhibition of bacterial growth was determined by absorbance reading at 600 nm (microplate reader Awarenees Technology). The MIC was defined as the lowest concentration of venom required to inhibit microbial growth. Each dilution series included control wells, which consisted of 50 µL of saline solution or antibiotic (chloramphenicol 6 µg µL-1). All assays were run by duplicates and three assays were performed.
RESULTS
Isolation, identification, and growth inhibition from Fusarium
The isolates of microorganisms showed a violet cotton colony. The microscopy analysis showed septate hyphae, macroconidia and microconidia compatible with Fusarium spp. The MALDI-TOF analysis indicated Fusarium oxysporum.
The inhibitory activity of venoms in agar plates against this microorganism was reduced and only doses of 1 mg/10 µL to B. asper showed inhibition halos of 8 mm (data not shown). P. nasutum did not shown inhibitory activity.
Isolation, identification, and growth inhibition from Xanthomonas
Two isolates of bacteria showed yellow mucus colonies, and Gram staining indicated gram negative rod shaped bacterium. Test of oxidase and catalase were negative and the MALDI-TOF analysis indicated one isolated for Xanthomonas axonopodis and another for Xanthomonas perforans (Tab. 1).
Table 1. Isolation and identification of Xanthomonas from Passiflora edulis f. edulis samples.
| Test | Isolate 1 | Isolate 2 |
|---|---|---|
| Strain morphological characteristics | Mucous yellow colony | Mucous yellow colony |
| Gram stain | Gram negative bacillus | Gram negative bacillus |
| Oxidase | Negative | Negative |
| Catalase | Negative | Negative |
| MALDI-TOF | Xanthomonas axonopodis | Xanthomonas perforans |
The inhibitory activity of venoms in agar plates against this microorganism was dependent on the species and doses used. The B. asper venom showed the highest activity against both bacterial species. Doses of 250 µg/10 µL produced inhibition halos of 20 mm for X. axonopodis and 17 mm for X. perforans, representing 80 and 70% inhibition, respectively, compared to the antibiotic used as control. In contrast, P. nasutum only showed 40 and 20% inhibition respectively at the same doses (Fig. 2).
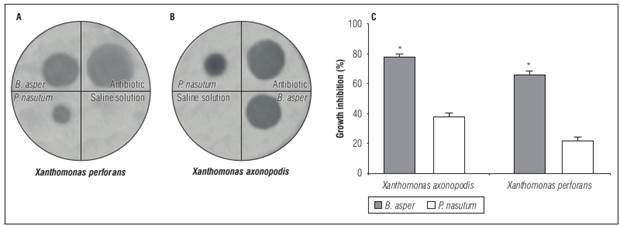
Figure 2. Antimicrobial activity in agar diffusion assay of B. asper and P. nasutum venom against Xanthomonas isolated from Passiflora edulis samples. A) and B) inhibition halos. C) inhibition percentage in relation to the inhibition halo showed by antibiotic used. Bars represent mean ± SD of duplicate * Represents statistically significant differences (P<0.05).
In the broth microdilution assay both venoms showed growth inhibition in all doses used (Fig. 3). However, the MIC to B. asper venom was 31.2 µg mL-1 while P. nasutum exhibited a MIC of 500 µg mL-1 (Tab. 2) against both bacteria. Furthermore, the venoms showed statistical differences (P<0.001) in the absorbance obtained in concentration below the MIC compared to the growth control. The positive control (chloramphenicol) completely inhibited growth at the concentration used.
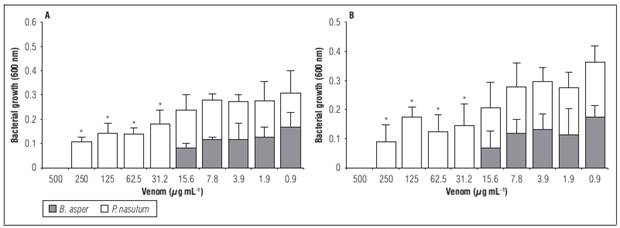
Figure 3. Antimicrobial activity in broth microdilution assay of B. asper and P. nasutum venom against Xanthomonas isolated from P. edulis f. edulis samples. A) X. perforans; B) X. axonopodis. Bars represent mean ± SD of triplicate, asterisk indicate statistically significant differences in antimicrobial activity of both venoms (P<0.05).
Table 2 Minimal inhibitory concentration (MIC) (in µg mL-1) of B. asper and P. nasutum venom against X. axonopodis and X. perforans. The MIC was lowest doses of venom required to inhibit totally microbial growth.
| Venom | Xanthomonas axonopodis | Xanthomonas perforans |
|---|---|---|
| B. asper | 31.2 | 31.2 |
| P. nasutum | 500 | 500 |
DISCUSSION
Snake venoms are complex mixtures of proteins and peptides and exhibit a wide range of pharmacological activities, and could be used to develop new treatments in different areas for example in agricultural sector. Plant diseases represent a growing problem concern for global food security, and their control is crucial to produce food, and others as flowers, fibers and biomaterials (Rahman et al., 2018). The annual losses of crops cause high economic impact on the food market. The most important pathogens that cause high damage to fruit and vegetable crops are bacteria and fungi (Rahman et al., 2018; Shah et al., 2022).
Every day many efforts are done to reduce bacterial and fungal infections that affect crop yield. Xanthomonas is a gram negative bacterial genus contains species. Occurrences of Xanthomonas diseases have been reported from multiple hosts worldwide and had caused major losses in different crops. X. axonopodis pv. passiflorae caused bacterial spot in passion fruit, it attacks the purple and yellow passion fruit as well as sweet passion fruit (Munhoz et al., 2011).
In this work, B. asper and P. nasutum venom demonstrated inhibitory activity against two Xanthomonas species. This bactericidal activity is consistent with previous reports of these venoms exhibiting efficacy against both gram-positive and gram-negative bacteria (Yu et al., 2010; Santamaría et al., 2005; Vargas et al., 2012). The MIC of B. asper venom was found to be lower compared to P. nasutum venom, which could be attributed to differences in the protein composition of their venoms (Alape-Girón et al., 2009; Lomonte et al., 2012).
Thus, the possibility of finding molecules that in the future allow the development applications in agricultural field for the control of this bacterium that causes losses in crops, such as purple passion fruit. Although they preliminary results it is not unthinkable, since there is other example of development of molecules from snake venom in other areas. Majority of the clinical and to date several snakes venom based treatments have been approved for cardiovascular diseases and other news uses currently being investigated in clinical trials as example, chronic pain, cancer and infections (Pérez-Peinado et al., 2020). In relation to infections disease several venoms had been assay and demonstrated activity against different microorganisms related with clinical pathologic, examples include enzymes and proteins as L-aminoacid oxidase (LAAO), metalloproteinases, desintegrins, lectins and antimicrobial peptides (cathelicidin). The mechanism of action included direct toxic action, free radical generation, induction of apoptosis, protease inhibitors, among others (Shamsi et al., 2016; de Barros et al., 2019; Pérez-Peinado et al., 2020).
The evaluation of snake venoms against microorganisms involved in plant pathologies is scarce. Toyama et al. (2006) evaluated a LAAO from Crotalus durissus cascavella (CascaLAO) against X. axonopodis pv. passiflorae and observed an inhibition of 50% growth using 35 µg mL-1. This activity was demonstrated through transmission electron microscopy, which revealed the rupture of the plasma membrane of microorganisms, leading to the leakage of cytoplasmic content and cell death. However, a PLA2 isolated from this venom only inhibited 9.8% their growth (Oliveira et al., 2008). The lectins CTC and CVX-like isolated from Crotalus durissus terrificus species inhibited 87.8% growth of this bacteria (Rádis-Baptista et al., 2006). Additionally, the venom and PLA2s from Bothrops jararacussu showed antibacterial effects on this bacterium, with the whole venom demonstrating better activity (Barbosa et al., 2005).
In the same way Gomes et al. (2005) obtained a peptide from Bothrops jararaca venom with antifungal activity over F. oxysporum and Colletotrichum lindemuthianum phytopathogenic fungi. However, the activity was minor in relation with other fungi evaluated. Similarly, viper venoms evaluated in this work demonstrated low activity over F. oxysporum isolated from purple passion fruit.
The discovery of new potent antimicrobial agents with different mechanisms of action remains of high priority. The expectation to find such agents in snake venoms increases every day. In this study, the venoms of B. asper and P. nasutum demonstrated inhibitory activity against X. perforans and X. axonopodis, two microorganisms that cause significant damage in Passiflora edulis f. edulis crops. Further studies to isolate the compound responsible for this action are desirable.
CONCLUSION
In this study, we evaluated the antimicrobial activity of venoms from B. asper and P. nasutum against isolates of Xanthomonas and Fusarium from Passiflora edulis f. edulis. The results showed MIC values of 31.2 and 500 µg mL-1 for B. asper and P. nasutum, respectively, against X. axonopodis and X. perforans species. The activity of both venoms on Fusarium isolates was low, with only B. asper showing activity at high doses. The significant antibacterial activity against Xanthomonas indicates the potential of these venoms as new sources for alternative treatments against this phytopathogens that cause considerable losses in agriculture, especially in passion fruit crops.















