INTRODUCTION
Oropouche virus (OROV) is one of arboviruses that affect the human health, and it is responsible for causing Oropouche fever (OF). The first registered case of OF was in 1955 from a worker in Trinidad and Tobago [1, 2]. In Brazil, the first isolated virus was in 1960 from the blood of a sloth (Bradypus tridactylus) captured in a forest area during the construction of the Belém-Brasilia highway [3]. OROV belong to the Orthobunyavirus genus and Peribunyaviridae family (order Bunyavirales) been it the only member of this group that is able to infect humans [2, 4]. This virus is transmitted to humans predominantly by the biting of Culicoides paraensis, a common and widespread American bloodsucking midge. The transmission of OROV has two distinct cycles: an epidemic urban cycle with humans as the primary vertebrate host, and a jungle cycle, where primates, sloths, and birds have been proposed as vertebrate reservoirs [2, 5-8].
The OF is an acute febrile illness characterized by an incubation period between 4 to 8 days. The fever can achieve 40 °C and the main symptoms include headache, astenia, chills, photophobia, dizziness and arthralgia. Besides those classic symptoms, have also been reported neurological manifestations such as meningism and meningitis [3, 9]. Structurally, the OROV is a spherical lipid-enveloped particle with 80-120 nm of diameter surrounded by helical nucleocapsid [10, 11]. The genome of virus is formed by three negative-sense, single-stranded RNA segments called large (L), medium (M) and small (S) that encodes the viral RNA-dependent RNA polymerase (RdRp), the viral surface glycoproteins (Gn and Gc) and the nucleocapsid protein (N), respectively [10]. Although that some stages of the OROV replication cycle are still unclear, currently is known that the viral particle can penetrate into cell by endocytosis mediated by clathrin-coated vesicles after adsorption at surface of host cell. Finally, the mature virus performs a lytic cycle by induction of apoptosis in host cell, which is observed 36 hours after initial infection [10].
The clinical diagnosis of OF is dubious due to the non-specificity of symptoms, which are also characteristic of other arboviruses as Dengue and Zika [12]. Laboratory diagnosis is based on classical and molecular virology techniques. The detection of the virus genome by polymerase chain-reaction (PCR) in the plasma or serum of patients is currently one of methods more widely employed. Furthermore, serological tests such as neutralization for plaque reduction, complement fixation, inhibition of hemagglutination, enzyme immunoassay and detection of IgM and IgG in convalescent sera are also routinely used as indirect methods to detection of virus [1, 12]. However, rapid tests based on immunoassays (such as Elisa or immunochromatography) are not yet available to the market to facilitate accurate diagnosis. Moreover, there are no studies proving the possibility of identification through fluids such as saliva and urine, which are possible in other viral infections [13]. Thus, in view of the difficulties related to the high costs and difficulties in carrying out the diagnostic tests currently available, many cases of OROV infection have been underreported. This context means that there is a lack of relevant epidemiological information, which supports the implementation of actions and measures of prevention and control of this disease.
The emergence and re-emergence of OROV in Central and South America have resulted in more than 30 outbreaks, which are characterized by infection rates exceptionally high, i.e., exceeding 40% ofpopulation affected [6, 11]. This arbovirus has been reported in Brazil, Peru, Panama, and Trinidad and Tobago, been that the majority the cases are concenter in Brazil. However, the epidemiological studies of OF conducted in Brazil are decentralized and focus mainly on isolates outbreaks and on suspected cases in different regions of the country. Thus, a detailed analysis regarding the epidemiology of OROV at the national level has not been conducted until the date. Herein, we aimed describe the overall incidence of OROV in Brazil and other South America countries through a systematic review of the data available in the biomedical literature.
METHODS
A systematic literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (Prisma) [14]. Pubmed/Medline, Scopus, Cochrane library, Lilacs, Scientific Electronic Library Online (SciELO), and Biblioteca Virtual em Saúde (BVS) databases were searched for articles in English, Portuguese or Spanish published until April 15, 2020. Indexed keywords from Medical Subject Headings (MeSH) and Descritores em Ciências da Saúde (DeCS) were used to build search strategies. The descriptor "Epidemiology" (or "Epidemiologia" in Portuguese) was combined with each of the following descriptors: "Oropouche fever" OR "Oropouche virus" OR "virus, Oropouche" OR "fever, Oropouche" OR "OROV." The connector AND was used between terms, according to the following example: "Epidemiology" AND ''Oropouche virus''. To avoid losing any possible study, the reference list of all included articles was manually screened to identify potential eligible studies.
The primary interest was finding data related to the past or recent exposure to OROV in countries from South America. The past exposure was considered as the positive serological evidence of contact by determination of IgG anti-OROV. For recent exposure, it was defined as a OROV case that tested positive using direct tests (i.e., identification of the genetic material of the virus by reverse-transcriptase polymerase chain reaction (RT-PCR) or isolation of the virus in cell culture and/or in neonate mice) or serological evidence (IgM anti-OROV) [12, 15]. Finally, the overall rate of exposure was considered the ratio between the number of recent and past cases and the total number of patients screened [16].
Review articles, notes, e-mails, editorials, letters, papers presented at scientific events and articles that had no original data were excluded. Articles were also excluded based on studies that: (I) included only cases of infections in animals by OROV; (II) did not report the nationality of the patients included and (III) identified the presence of OROV, but not describe the diagnostic method used to detect the viral infection.
During the selection, two independent researchers (R.S.P. and J.F.P.) have analyzed the articles according to the inclusion and exclusion criteria. Initially, the title, abstract and keywords were read to identify and pre-select articles of interest. Subsequently, pre-selected articles were read completely in order to confirm their suitability for this review. Discrepancies in decisions between the two researchers were resolved by discussion with a third researcher (L.G.F.C.), with a consensus determining if the article would be included [14]. Selected articles were submitted to an integral analytical reading to identify and extract the variables of interest which were summarized in table 1 for further critical analysis, interpretation and discussion of the data.
The quality of the selected studies was evaluated using the Newcastle-Ottawa scale [17]. Because the primary outcome was the rate of exposure to OROV in South America, the parameters "selection of the non-exposed cohort", "demonstrated that the outcome of interest was not present at the start of the study" and "comparability between cohorts" did not apply to our analysis. Thus, a maximum of five stars could be assigned to each study, and scores were assigned to classify the quality of the studies. Those with four or more stars were considered high quality, with three stars, moderate, and two or fewer stars, low quality.
RESULTS
The articles were selected based on the use of methodology with pre-defined criteria in the studies, as shown in figure 1. 115 articles were identified by searching the selected databases been 11 from Lilacs; 11, SciELO; 20, Scopus; 35, BVS and 38 from PubMed. Duplicate studies (n= 46) were excluded resulting in 69 studies that were selected for the full text reading. From those 69 articles, 9 were excluded because OROV was not the subject of study and 42 were excluded based on the criteria indicated in figure 1. As a result, 18 studies were selected as eligible to compose this review on epidemiological aspects of OROV. Table 1 presents a summary of the selected studies as well as their main information.
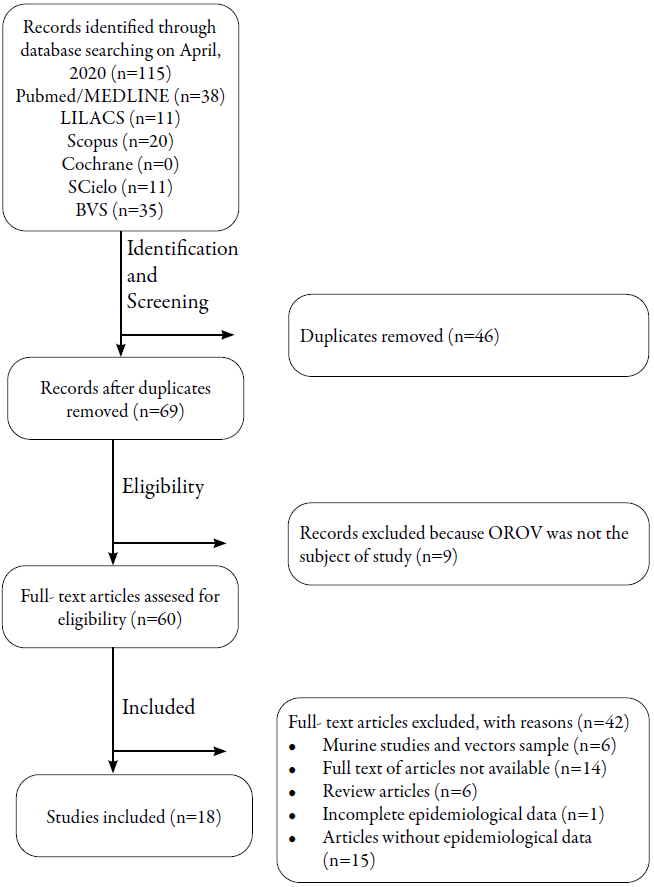
Figure 1: Flowchart of the selection of articles for the systematic review according to the Prisma criteria.
Table 1 Main clinical, laboratory and epidemiological information of the included studies.
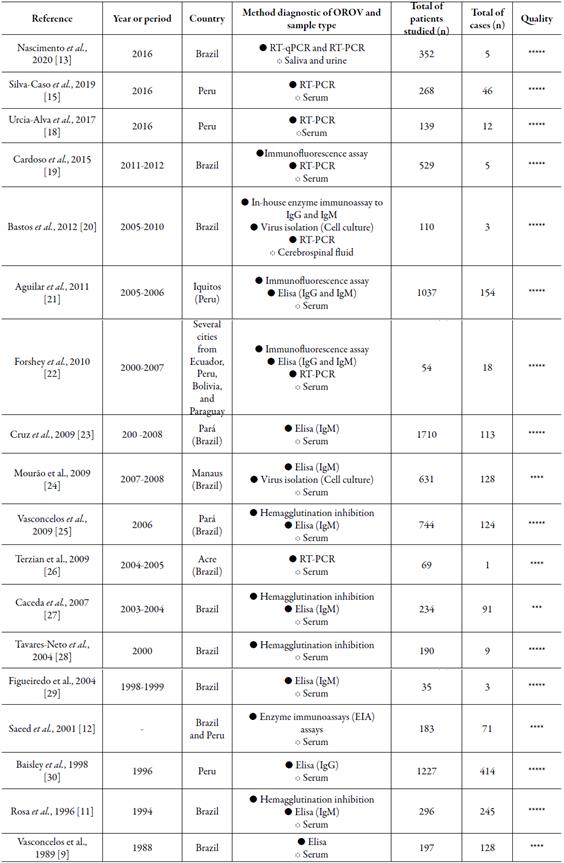
***** or **** = high quality; *** = moderate quality
The overall quality of the selected studies was good, with high (17/18; 94.4%) or moderate (1/18; 5.5%) methodological quality according to the Newcastle-Ottawa scale.
The studies were published between 1989 and 2020. Therefore, most of these studies date back to 2010, which demonstrates the importance of verifying the epidemiological distribution of O ROV in last decade. The pioneering study on O ROV in Brazil was in 1988, in the states of Goiás and Maranhão, based on the detection of antibodies in patients who had symptoms of fever OROV [9].
Table 1 shows that most studies were carried out in Brazil (12/18; 66.66%) and Peru (5/18; 27.77%). In turn, only one study has considered OROV research in samples from patients residing in the Ecuador (1/18; 5.55%), Bolivia (1/18; 5.55%) and Paraguay (1/18; 5.55%). The testing for the OROV occurred mainly by the collection and analysis of serum from the participants. Serum samples of patients were evaluated by hemagglutination inhibition, enzyme immunoassays (EIA), immunofluorescence and Elisa for identification of specific anti-OROV antibodies. Moreover, reverse transcriptase associated with polymerase chain-reaction (RT-PCR) and virus isolation on cell cultures were employed in some studies as a direct method of OROV identification. In addition, saliva and urine samples were evaluated by RT-PCR and Elisa and cerebrospinal fluid samples evaluated by RT-PCR. Of the 8005 samples analyzed, 1570 tested positive for the presence of OROV thus accounting a general exposure rate in South America of 19.61%.
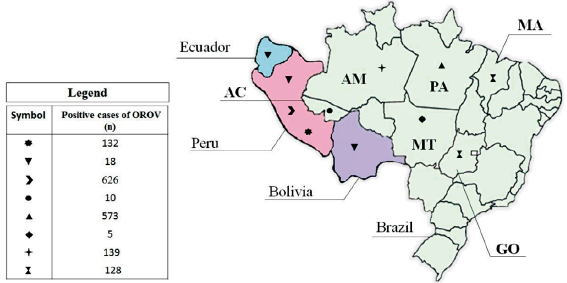
According to figure 2, 5 countries in South America reported cases of OROV infection, namely Brazil, Peru, Bolivia, Paraguay, and Ecuador. Most of the patients studied are from Brazil (5097/8005; 63.67%), followed by Peru (2671/8005; 33.37%). Brazil is responsible for more than half of the cases of OROV identified in South America (855/1570; 54.46%), however Peru has the highest rate of exposure to the virus among the countries of the continent (23.43% of frequency in Peru vs. 16.77% of frequency in Brazil). It is worth mentioning that two studies together counted data from patients from different countries, which made the data processing unfeasible in the stratified analysis by region.
DISCUSSION
The oropouche fever (OF) is an arthopod-borne arboviral disease which depends on several factors to emerge, such as weather conditions, availability of suitable breeding sites and increased migration [31]. Thus, changes in ecology may lead to the increase of OROV cases due to vegetation loss, which can affect the transmission cycle of this disease by favoring the migration of the vector and involved hosts [15, 31]. Romero-Alvarez et al. [32] found in their study that the OROV cases took place at localities that tended to have experienced vegetation loss before the outbreak in comparison with case-free sites. Interestingly, the Brazil that was the country that lost the most flowers in the last decade (2010-2020) according to Food and Agriculture Organization (FAO) [33] presents the largest number of cases of OROV in the South America as shown in this review. In Brazil, the increase of agricultural activities is also an important factor, because it raises the availability of organic matter, which can lead to an increase of the breeding of the vectors midges that preferentially breeds in decomposing organic matter [31].
The treatment for OF is symptomatic and there is no vaccine for its prophylaxis in humans [10]. Therefore, the prevention is the best option, and it is based on control or eradication measures for the arthropod vectors by the eradication of breeding sites in order to reduce midge populations and good agricultural practices, and personal protection to prevent the mosquito bites [10].
The pattern of occurrence of the OF, where cases take place on tropical and subtropical countries, emphasizes the importance of the climate on this issue. This happens because the vector is favored by hot and humid climate [10, 15], which justifies the profile found in this review that Amazonian countries, such as Brazil and Peru, are the main ones affected by arboviroses. However, considering that environmental and climate changes, and extensive animal and human population migration are a global phenomenon, it will not be surprising for OROV to spread outside South America in the near future [10].
OROV is, after dengue virus, the most frequent arbovirus in Brazil and it has an important social and economic impact [10]. The consequences of the OF outbreaks in Amazônia (Brazil) are well known, due to its massive transmission in a short period [34]. It is estimated, based on previously studied outbreaks, that over 300 000 people were infected with OROV in Amazônia [35]. Considering that the virus is circulating quickly around Brazilian cities, especially in the northern cities, it is important to provide more information and data about the OROV and OF. It is also necessary to invest on the diagnosis and the prevention measures in order to prevent another epidemic, such as Dengue and Zika.
According to the results obtained in this review, serological assays (i.e., hemagglutination inhibition, IgM/IgG capture enzyme-linked immunosorbent assay [Elisa]) were the main methods used to diagnose and confirm OROV infection, followed by RT-PCR, and the viral isolation on cell culture (C6/36 or Vero cells). OROV infections can be diagnosed based on apparent pathology, presence of the vector, serological and molecular evidence of OROV exposure, and isolation of the virus in cell culture or neonate mice [12, 15]. An accurate and timely diagnosis of OROV infections is essential for choosing treatment strategies that limit the infection and its joint complications. However, the clinical diagnosis of OROV is difficult because its symptoms overlap with other arboviruses [15]. Furthermore, there are important challenges for OROV laboratory diagnosis, partly due to the narrow time-frame for detecting the viral RNA and cross-reactive antigens with antibodies against other virus such as Dengue, Zika and Chikungunya [12, 15]. For this reason, combinations of techniques are needed to obtain an appropriate differential diagnosis of which is used by many of included studies.
The socioeconomic status of the population can be related to the incidence of OROV cases because the housing conditions and the basic sanitation are important matters to the origination of the vector midges. Similarly, to the Aedes aegypti (dengue vector), the Culicoidesparaensis (major OF vector) is favored by humidity and the breending occurs in flooded areas [10]. Besides that, Culicoides paraensis breeding is also favored by the presence of decomposing organic matter and animal feces, which explains the high incidence of cases in rural areas [10, 31].
According to the most recent epidemiological bulletin provided by the health surveillance in Brazil on OF, 26 cases of this disease were detected in four northern states of Brazil until the 35th week of 2019 and no information about the deaths related to the OF was reported. Comparing this data with the Dengue, Zika and Chikungunya incidence, the most recent epidemiological bulletin on these arboviruses were conducted until the 21st week of 2020. Regarding to Dengue, 778 400 cases were reported as probable cases, which leads to an incidence rate of 370.4 cases per 100 000 inhabitants. Although the cases are widespread throughout the country, the central west region presented the highest incidence rate (928.1 cases per 100 000 inhabitants). Regarding to Chikungunya, 34 751 cases were reported as probable cases, with an incidence rate of 16.5 cases per 100 000 inhabitants. The northeast and southeast regions presented the highest incidence rates, 29 cases/100 000 inhabitants and 18.2 cases/100 000 inhabitants, respectively. Regarding to Zika, 3509 cases were reported as probable cases, with an incidence rate of 1.7 cases per 100 000 inhabitants and the northeast region presented the highest incidence rate (3.6 cases/100 000 inhabitants) [36, 37].
Despite the difference between the incidence rate of OROV and the other arboviruses, the World Health Organization (WHO) emphasized the epidemic potential of arboviruses, which includes OROV. The studies referenced in this review showed that this disease could be a threat to the public health, once it has been described in areas that was not previously identified, which highlights the spreading ability of the OROV [15].
It is important to emphasize that patients may be asymptomatic and when they have symptoms, those are similar to the symptoms of other arboviral diseases such as West Nile, Yellow Fever, Dengue, Chikungunya, Zika and Mayaro [1, 10, 15]. Thus, it is likely that OF cases are unreported or misdiagnosed [38], and there are no published reports of OROV-related human deaths or autopsies, so very little information about the pathogenesis of OROV human infections is available [39]. The OF can enroll neurological manifestations, clinic defined as meningitis or meningismus, which was observed during the 1980 outbreak in Pará (Brazil) [34]. Therefore, there is a difficulty in the acquisition of actual data, and it is difficult to predict how this disease could spread and what could be the public health implications around the world.
CONCLUSION
An epidemiological overall status of the OF was provided with this review, and it showed that the OROV could represent a potential threat to a new epidemic in the near future. Here we show that the general rate of exposure to OROV is in South America is of 19.61%, and that Brazil and Peru are the main reservoirs of the disease on the continent. Therefore, is important to make the differential diagnosis of it and the epidemiological bulletin provided by the health surveillance in South America countries should be more accurate about the incidence rates. Those measures would provide the proper tools to predict the real risk associated to this disease and the affected countries would be able to be avoid a possible epidemic.