Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Colombian Journal of Anestesiology
versión impresa ISSN 0120-3347
Rev. colomb. anestesiol. v.38 n.4 Bogotá oct./dic. 2010
Investigación Científica y Tecnológica
The Use of Profound Sedation Assisted by the Anesthesiologist in Pediatric Patients Undergoing MRI
Jorge Andrés Delgado*, Pedro Abad**, Gabriel Jaime Angel***, Juan Fernando Llano****, Francisco Javier Gómez*****, Víctor Daniel Calvo******
* MD, especialista en radiología. Director científico e investigador de la Fundación Instituto de Alta Tecnología Médica de Antioquia. Medellín, Colombia. jorge.delgadojandres@gmail.com, investigacion@iatm.com.co.
** MD, especialista en radiología. Investigador de la Fundación Instituto de Alta Tecnología Médica de Antioquia. Medellín, Colombia.
*** Especialista en enfermería cardiovascular. Jefe de Enfermería e investigador de la Fundación Instituto de Alta Tecnología Médica de Antioquia. Medellín, Colombia.
**** MD, especialista en radiología. Investigador de la Fundación Instituto de Alta Tecnología Médica de Antioquia. Medellín, Colombia.
***** Profesor titular y jefe del Servicio de Anestesiología y Reanimación de la Universidad de Antioquia e investigador de la Fundación Instituto de Alta Tecnología Médica de Antioquia. Medellín, Colombia.
****** Profesor de Bioestadística de la Facultad de Medicina de la Corporación Universitaria Remington e investigador de la Fundación Instituto de Alta Tecnología Médica de Antioquia. Medellín, Colombia.
Recibido: mayo 10 de 2010. Enviado para modificaciones: mayo 20 de 2010. Aceptado: junio 30 de 2010.
Summary
Objetive. Obtaining diagnostic quality images in the pediatric population using magnetic resonance imaging, requires the use of deep sedation assisted by the anesthesiologist to ensure the total immobility of the patient for an adequate examination.
Objective. To describe the use of deep sedation assisted by an anesthesiologist in magnetic resonance studies for the pediatric population.
Methodos. Observational study of a series of cases. 113 randomly selected MRI scans, with assisted sedation by an anesthesiologist in pediatric patients aged less than 15, treated at the Fundación Instituto de Alta Tecnología Médica de Antioquia in 2009. The inter-observer consistency was evaluated in 84 examinations with their corresponding series of images.
Results. Average sedation time for the most common MRIs were are follows: cerebral MRI, 45.2 ± 12.4 minutes; cerebral with contrast, 46.3 ± 16.7 minutes; cardiac, 96 ± 24.1 minutes; cerebral angiography, 60 ± 16.8 minutes and cerebral-total spine, 76.3 ± 32 minutes. No significant gender-adjusted differences were found (p > 0.05). The sedatives used for these examinations were: midazolam, ketamine, propofol, chloral hydrate and fentanyl. Excellent inter-observer consistency was found in terms of the reliability of the series of images of deep sedation patients (Kappa > 0.9).
Conclusions. Sedation assisted by an anesthesiologist is considered a procedure with a low rate of complications that can be used more often in the pediatric population for obtaining diagnostic quality images in patients with co-morbidities and in complex MRI procedures.
Keywords: Deep sedation, magnetic resonance imaging, psychotropic drugs, pediatrics (Source: MeSH, NLM)
Resumen
Introducción. Para la obtención de imágenes con calidad diagnóstica en la población pediátrica por medio de la resonancia magnética es necesario usar la sedación profunda asistida por un anestesiólogo, dado que ésta garantiza la inmovilidad completa del paciente para el adecuado desarrollo del examen.
Objetivo. Describir el uso de sedación profunda asistida por un anestesiólogo en los estudios de resonancia magnética para población pediátrica.
Métodos. Estudio observacional de serie de casos; se eligieron aleatoriamente 113 exámenes de resonancia magnética asistida por anestesiólogo en pacientes menores de 15 años de edad, atendidos en la Fundación Instituto de Alta Tecnología Médica de Antioquia en el 2009; y para la concordancia entre observadores se evaluaron 84 exámenes con sus respectivas series de imágenes.
Resultados. Los tiempos promedio de sedación de las resonancias magnéticas más comunes fueron: cráneo simple, 45,2 ± 12,4 minutos; cráneo contrastado, 46,3 ± 16,7 minutos; cardiaca, 96 ± 24,1 minutos; angio de cráneo, 60 ± 16,8 minutos, y cráneo-columna total, 76,3 ± 32 minutos. Al ajustar por sexo no se hallaron diferencias significativas (p > 0,05). Los medicamentos sedantes empleados para estos exámenes fueron: midazolam, ketamina, propofol, hidrato de cloral y fentanyl. Respecto a la fiabilidad de las series de imágenes de los pacientes con sedación profunda, se halló una excelente concordancia entre observadores (Kappa > 0,9).
Conclusión. Se considera la sedación asistida por un anestesiólogo un procedimiento con baja tasa de complicaciones, el cual puede ser usado con mayor frecuencia en la población pediátrica para la obtención de imágenes con calidad diagnóstica en pacientes con comorbilidades y en procedimientos de resonancia magnética complejos.
Palabras clave: Sedación profunda, imagen por resonancia magnética, psicotrópicos, pediatría (Fuente: DeCS, BIREME)
Introduction
Magnetic Resonance Imaging (MRI) plays a key role in the diagnosis of pathologies involving the central nervous system, the cardiovascular system and the musculoskeletal system. With the advent of more powerful equipment and new imaging sequences, non-invasive techniques have been developed in the last 15 years to assess these body systems, particularly in the pediatric population (1-3). Obtaining high quality MRIs requires total immobility of the patient during the procedure – a rather difficult task with pediatric patients due to their young age or to their clinical condition. Other relevant factors are the type of MRI according to the clinical indication, the claustrophobic environment created by the limited space inside the machine or the noise produced by the radiofrequency waves needed to obtain the images. Hence the need for deep anesthesia assisted sedation in order to achieve diagnostic quality examinations in pediatric patients and to ensure the confidence of parents. (4-8). Until 1985 there were no guidelines for the use of sedation and anesthesia (4); however, due to the increasing number of reports about complications, the American Academy of Pediatrics (AAP) developed the first guidelines for elective sedation. Later, the American Society of Anesthesia (ASA) also developed its own guidelines that were then adopted by the AAP (4).
Currently, MRI has proven its diagnostic value, giving rise to a higher demand by clinical specialists and to an interdisciplinary effort with the anesthesia department, particularly for pediatric patients (9). However, the anesthesiologist must be thoroughly acquainted with the environment where the MRI is done in order to ensure the safety of the patient, of the assisting staff and his/her own safety, in addition to appropriate sedation and recovery after the procedure (1).
The objectives of deep sedation for doing an MRI are: preventing voluntary or involuntary movements, managing the intolerance to the procedure, adequate immobilization, reduce the risk of cardiovascular arrest due to the baseline conditions of the patient, meet the need to constantly monitor for hemodynamic instability or for the risk of airway obstruction and apnea (10,11). Failure to do an MRI because of poor deep sedation is not only detrimental to the patient but also causes delays in administering appropriate treatment and translates into higher medical care costs (12).
Materials and methods
There are hardly no comparative studies regarding the effects of decreasing doses of LBP in terms of the duration of the motor block and the quality of the intra-operative analgesia, and it is not clear whether there is a true clinical difference in terms of the advantages of the spinal use of low doses of this s-enantiomere. The objective of this comparative randomized clinical trial was to assess the clinical profile and motor block probability through time using three different doses of 0.75 % hyperbaric LBP (HLBP) (7,5, 9,37 and 11,25 mg) with a short (5 minutes) unilateral spinal technique, recreating the situation of many operating rooms where the block is performed in the OR and where the effective management of the induction time is critical during the highly-demanding periods of outpatient unilateral arthroscopic procedures in the knee.
A descriptive observational study of a series of cases was undertaken in pediatric patients under 15 years of age sedated by an anesthesiologist for MIR in 2009 at the Fundación Instituto de Alta Tecnología Médica de Antioquia (IATM), located in Medellín, Colombia. 113 MR examinations were selected through random sampling with a 95 % Confidence Interval and a random error probability of 0.05. 84 examinations with their corresponding images were selected using the finite population random sample correction factor.
Upon revision, approval and authorization by the ethics committee of the IATM Foundation, the clinical records of the patients were accessed and compiled in the Radiology Information System (RIS) and in the Picture Archiving and Communication Systems (PACS). In accordance with the International Declaration of Helsinki, The Belmont Report and Resolution 8430 (1993) of Colombia, this research was rated risk-free.
Establishment of an Institutional Deep Sedation Protocol
When the patient arrives at the MRI department, the nursing staff proceeds to complete the clinical forms. The vital signs are measured and the doctor determines the type of sedation to be used for the MR procedure.
If the patient is a minor and weights under 30 kg, chloral hydrate is administered by mouth, at a dose of 50 to 75 % of body weight, 15 to 20 minutes prior to the MRI; vein catheterization is performed and the patient is provided with all the necessary comfort to induce sleep. Pulse oximetry is monitored and low dose oxygen is administered (1 to 2 liters per minute), making sure that the oxygen saturation never drops below 90 %. Once the patient falls asleep, he/she is transferred to the room and placed on the MR table; the heart rate, blood pressure and pulse oximetry are monitored. Oxygen is supplied through a nasal cannula at a rate of 1 to 3 liters per minute; if necessary, deep sedation is administered I.V. with low dose midazolam (0.05 to 0.1 mg/kg), mixed with low dose ketamine (0.5 to 1 mg/kg). No Fentanyl is administered to patients less than 2 years old. However, patients over 2 years old and a body weight above 30 kg, receive midazolam and ketamine at the above-mentioned doses, together with fentanyl (0.5 to 1 μg/kg of body weight). Propofol is co-administered to both age groups with 2 % lidocaine SE (0.5 to 1 mg/kg). Finally, the MRI is performed under the supervision of the anesthesiologist.
Analysis Plan
In order to assess the quality of the MR examination two expert radiologists acting as anonymous observers were randomly selected for the classification using a series percentage indicator with movement artifacts.
The clinical records were used to verify the patient’s age, weight, indication for the examination and the type of MR performed. The sedation time was established using the sedation records of the IATM Foundation that reports the time in minutes from the start of the sedation procedure until its completion. The same form was used to check the drugs and doses administered to the patient and the anesthesiologist recorded the times of administration of the drug, the doses and frequency of administration.
Statistical analysis
The descriptive analysis was based on absolute, percentage and summary measurements. The normal distribution criterion for age, sedation time and drug dose was determined with the Kolmogorov-Smirnov test; the length of time under sedation, MR type and drug administered analysis was based on the t-student test. In terms of the different drug dose analysis according to age, Mann Whitney’s U test or Kruskal-Wallis test were used, while the inter-observer consistency was determined using Cohen’s Kappa index. p < 0.05 was considered significant.
The analysis of the data was done using the Aabel 3.0 20/20 statistical software.
Results
Demographic and clinical aspects
This study reviewed 113 pediatric patients clinical records of which 53 were ≥ 2 years old with an average of 5.1 ± 2.9 years; 55 infants with an average age of 7.9 ± 5.4 months and 5 neonates of 16.0 ± 8.7 days old. 61.1 % of the patients were males (n = 69).
In terms of the indications for deep sedation, 46.9 % (n = 53) were due to neurologic disorders resulting from growth retardation and related syndromes; 13.3 % (n = 15), were due to seizures; 9.7 % (n = 11) were due to related pathologies, difficult airway and cardiopulmonary disease; 7.1 % (n = 8) due to extreme age (≤1 month); 6.2 % (n = 7), due to prolonged MRI time exceeding 90 minutes. The rest was due to involuntary movements (n = 6), pain (n = 4), inadequate previous sedation (n = 2) and 6.2 % (n = 7) other causes such as difficulty to lie in decubitus position, metabolic acidosis and risk of bronchoaspiration.
With regards to the type of MR, 53.1 % (n = 60) were simple cerebral MRI; 14.2 % (n = 16), cerebral MRI with contrast; 8.9 % (n = 10), cardiac; 6.2 % (n = 7) cerebral angiography; 3.5 % (n = 4), abdomen angiography; 3.5 % (n = 4), cerebral and total spine and 10.6 % (n = 12), others: abdomen with contrast (n = 3), total spine (n = 3), cholangiography (n = 3), neck with contrast (n = 1), renal (n = 1) and lumbar spine (n = 1).
Characterization of time under deep sedation, type or MRI, drugs and doses administered
The most common average deep sedation times of the MRI procedures were: simple cerebral, 45.2 ± 12.4 minutes; cerebral with contrast, 46.3 ± 16.7 minutes; cardiac 96 ± 24.1 minutes; cerebral angiography, 60 ± 16.8 minutes and cerebral and total spine 76.3 ± 32 minutes; adjustment by gender did not produce any significant differences in the sedation time according to the type or MR (p > 0.05). The most frequent seda tives used in pediatric MRI were: midazolam (n = 81), 71.7 %; ketamine (n = 76), 67.3 %; propofol (n = 59), 52.2 %; fentanyl (n = 47), 41.6 %; chloral hydrate (n = 39), 34.5 %; mixture of midazolam, ketamine and fentanyl (n = 43), 38.1 %; mixture of midazolam and ketamine (n = 32), 28.3 %.
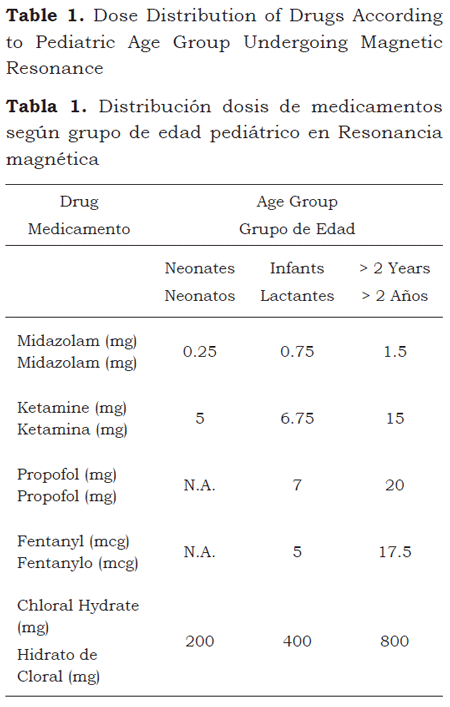
When adjusting by age group, 50 % of the neonates weighted 2,900 g or less; infants, 7,000 g, and patients over 2 years of age, 16,000 g. The median dose of the drugs used for the MRI was reported on the basis of these weights (table 1).
Significant differences were found when comparing the sedation times with chloral hydrate versus the other drugs: midazolam, ketamine, propofol, fentanyl and the combination of midazolam, ketamine and fentanyl (p < 0.0001, respectively); no significant differences were found between chloral hydrate and the midazolam and ketamine combination (p = 0.2093) (table 2).
Complications
Five cases resulted in complications: two due to increased secretions; one due to bronchospasm, another due to allergic reaction to the drugs and the fifth one because of a difficult airway requiring intubation. The drugs administered to these patients were: midazolam, fentanyl and ketamine (n = 3) and propofol (n = 5); four of the patients were over 2 years old and the other was an infant. The type of MR and the sedation time these patients required were: cerebral with contrast, 3 patients (none of them with sedation times differences vs. the non-complicated); simple cerebral, 1 patient (with a sedation time differential vs. the non-complicated) and cardiac MR, 1 patient (no sedation time differential vs the average, non-complicated cardiac MRIs). There were no fatalities from these complications.
Quality of Images
Of the 84 MR examinations with their respective series of images (randomly selected using correction factor and on the basis of the series of movement artifacts) Cohen’s Kappa index resulted in a 0.661 value between two observers with varying years of experience (14 and 4 years); however, when assessing the new index with a new observer with the same number of years of experience (14 years), Cohen’s Kappa index was 1.0, showing and excellent inter-observer consistency with regards to the reliability of the series of diagnostic images, when the observers had similar experience.
Discussion
The main purpose of doing an MRI under deep sedation assisted by an anesthesiologist is to obtain images for an accurate diagnosis of the pathology, basically images with no movement artifacts (4). The use of sedation for MRI procedures is much more frequent in pediatric patients as compared to adults because pediatric patients are subject to special conditions such as associated co-morbidities, claustrophobia, decubitus intolerance and indications due to age related poor collaboration (3,10,11).
Logically, anesthesia assisted deep sedation is not intended to raise the risk of an examination considered to be harmless, despite some potential risks derived from the electromagnetic energy inside the machine (3,13); likewise, the American Society of Anesthesiology issued a report in 2009 about anesthesia care in MR for obtaining diagnostic images (14). There were 5 (4.4 %), adverse events in our study that were managed immediately and with no consequences for the patients who were discharged in the same conditions as they were admitted. There were no fatal complications, nor significant morbidities and there was no need to interrupt the imaging procedure due to the occurrence of symptoms (2,6,11).
Anesthesiologists have different drug options depending on the length of time scheduled for the procedure and the depth required to ensure immobility and as much as possible, preventing complications, mainly airway related complications (15-21). These time periods are extremely useful to establish targeted protocols and to know in advance the length of time of the examination. This knowledge is very valuable for the anesthesiologist and for the rest of the team involved with the MRI procedure (14). Non of the patients were excluded due to underlying pathologies or extreme age (prematurity) and no significant differences were found as to gender, sedation times according to the type of MRI, drugs used or rate of complications.
The evaluation of the examinations of the randomized group with correction factor rendered high quality images and none of the MR studies showed movement artifacts that could hinder its interpretation. This shows the benefits of deep sedation when interpreting MRIs. The Cohen’s Kappa index used to compare inter-oberver diagnostic evaluation showed an excellent concordance; it should be emphasized however that both radiologists were “blind” to the clinical characteristics of the patients.
It must be highlighted that this is our first experience in developing a deep sedation protocol for the pediatric population, assisted by an anesthesiologist. This survey will provide us with better tools when making a decision about these types of procedures, the length of time required, the drugs to be used and any potential complications.
Conclusion
Deep sedation assisted by an anesthesiologist for magnetic resonance imaging, represents a valuable tool to ensure the safety of the procedure and high quality diagnostic images in the pediatric population, regardless of age, of any associated co-morbidities or the period of time the patient has to be immobilized inside the machine.
REFERENCES
1. Odegard K, DiNardo J, Tsai-Goodman B, Powell A, Geva T. Laussen P. Anaesthesia considerations for cardiac MRI in infants and small children. Paediatr Anaesth. 2004;14(6):471-6.
2. Kannikeswaran N, Mahajan PV, Sethuraman U. Sedation medication received and adverse events related to sedation for brain MRI in children with and without developmental disabilities. Paediatr Anaesth. 2009;19(3):250-6.
3. Bryson EO, Frost EA. Anesthesia in remote locations: radiology and beyond, international anesthesiology clinics: CT and MRI. Int Anesthesiol Clin. 2009;47(2):11-9.
4. Serafini G, Ongaro L, Mori A, Rossi C, Cavalloro F, Tagliaferri C, et al. Anesthesia for MRI in the paediatric patient. Minerva Anestesiol. 2005;71(6):361-6.
5. Mallory MD, Baxter AL, Kost SI and the Pediatric Sedation Research Consortium. Propofol vs pentobarbital for sedation of children undergoing magnetic resonance imaging: results from the Pediatric Sedation Research Consortium. Paediatr Anaesth. 2005;19(6):601-11.
6. Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell D. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth. 2000;84(6):743-8.
7. De Sanctis Briggs V. Magnetic resonance imaging under sedation in newborns and infants: a study of 640 cases using sevoflurane. Paediatr Anaesth. 2005;15(1):9-15.
8. Beauve B, Dearlove O. Sedation of children under 4 weeks of age for MRI examination. Paediatr Anaesth. 2008;18(9):892-3.
9. Melloni, C. Anesthesia and sedation outside the operating room: how to prevent risk and maintain good quality. Curr Opin in Anaesthesiol. 2007;20(6): 513-9.
10. Lawson GR, Controversy: Sedation of children for magnetic resonance imaging. Arch Dis Child. 2000;82(2):150-3.
11. Fogel M, Weinberg P, Parave E, Harris C, Montenegro L, Harris M, et al. Deep sedation for cardiac magnetic resonance imaging: a comparison with cardiac anesthesia. J Pediatr. 2008;152(4):534-9.
12. McBrien ME, Winder J, Smyth L. Anaesthesia for magnetic resonance imaging: a survey of current practice in the UK and Ireland. Anaesthesia. 2000;55(8):737-43.
13. Litt L, Cauldwell CB. Being extra safe when providing anesthesia for MRI examinations. Anesth Saf Newsletter. 2002;66:6.
14. American Society of Anesthesiologists. Practice advisory on anesthetic care for magnetic resonance imaging. Anesthesiol. 2009;110(3):459-479.
15. American Academy of Pediatrics, Committee on Drugs. Guidelines for the elective use of conscious sedation, deep sedation and general anesthesia in pediatric patients. Pediatrics. 1985;76(2):317-21.
16. Ramírez M, Martínez O, Flores R , Pineda L. Ketamina oral para evitar en los niños el dolor por procedimientos de diagnóstico o de tratamiento. Rev Mex Pediat. 2001;68(2):48-51.
17. Rao CC, Krishna G. Anaesthetic considerations for magnetic resonance imaging. Ann Acad Med Singapore. 1994;23(4):531-5.
18. Gozal D, Drengrer B, Levin PD, Kadari A, Gozal Y. A pediatric sedation/anesthesia program with dedicated care by anesthesiologists and nurses for procedures outside the operating room. J Pediatr. 2004;145(1):47-52.
19. Fernández I, Galán C, Torre C. Comparación de ketamina-midazolam con propofol-midazolam para sedación y analgesia en pediatría. Bol Pediatr. 2000;40(171):19-23.
20. Usher A, Kearney R, Tsui B. Propofol total intravenous anesthesia for MRI in children. Paediatr Anaesth. 2005;15(1):23-8.
21. Rashed AH, Shayevitz JR. Deep sedation wih propofol for children undergoing ambulatory magnetic resonance imaging of the brain: Experiencie from a pediatric intensive. Pediatric Critical Care Medicine. 2003;4(4):454-8.
1. Odegard K, DiNardo J, TsaiGoodman B, Powell A, Geva T. Laussen P. Anaesthesia considerations for cardiac MRI in infants and small children. Paediatr Anaesth. 2004;14(6):4716. [ Links ]
2. Kannikeswaran N, Mahajan PV, Sethuraman U. Sedation medication received and adverse events related to sedation for brain MRI in children with and without developmental disabilities. Paediatr Anaesth. 2009;19(3):2506. [ Links ]
3. Bryson EO, Frost EA. Anesthesia in remote locations: radiology and beyond, international anesthesiology clinics: CT and MRI. Int Anesthesiol Clin. 2009;47(2):119. [ Links ]
4. Serafini G, Ongaro L, Mori A, Rossi C, Cavalloro F, Tagliaferri C, et al. Anesthesia for MRI in the paediatric patient. Minerva Anestesiol. 2005;71(6):3616. [ Links ]
5. Mallory MD, Baxter AL, Kost SI and the Pediatric Sedation Research Consortium. Propofol vs pentobarbital for sedation of children undergoing magnetic resonance imaging: results from the Pediatric Sedation Research Consortium. Paediatr Anaesth. 2005;19(6):60111. [ Links ]
6. Malviya S, VoepelLewis T, Eldevik OP, Rockwell D. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth. 2000;84(6):7438. [ Links ]
7. De Sanctis Briggs V. Magnetic resonance imaging under sedation in newborns and infants: a study of 640 cases using sevoflurane. Paediatr Anaesth. 2005;15(1):915. [ Links ]
8. Beauve B, Dearlove O. Sedation of children under 4 weeks of age for MRI examination. Paediatr Anaesth. 2008;18(9):8923. [ Links ]
9. Melloni, C. Anesthesia and sedation outside the operating room: how to prevent risk and maintain good quality. Curr Opin in Anaesthesiol. 2007;20(6): 5139. [ Links ]
10. Lawson GR, Controversy: Sedation of children for magnetic resonance imaging. Arch Dis Child. 2000;82(2):1503. [ Links ]
11. Fogel M, Weinberg P, Parave E, Harris C, Montenegro L, Harris M, et al. Deep sedation for cardiac magnetic resonance imaging: a comparison with cardiac anesthesia. J Pediatr. 2008;152(4):5349. [ Links ]
12. McBrien ME, Winder J, Smyth L. Anaesthesia for magnetic resonance imaging: a survey of current practice in the UK and Ireland. Anaesthesia. 2000;55(8):73743. [ Links ]
13. Litt L, Cauldwell CB. Being extra safe when providing anesthesia for MRI examinations. Anesth Saf Newsletter. 2002;66:6. [ Links ]
14. American Society of Anesthesiologists. Practice advisory on anesthetic care for magnetic resonance imaging. Anesthesiol. 2009;110(3):459479. [ Links ]
15. American Academy of Pediatrics, Committee on Drugs. Guidelines for the elective use of conscious sedation, deep sedation and general anesthesia in pediatric patients. Pediatrics. 1985;76(2):31721. [ Links ]
16. Ramírez M, Martínez O, Flores R , Pineda L. Ketamina oral para evitar en los niños el dolor por procedimientos de diagnóstico o de tratamiento. Rev Mex Pediat. 2001;68(2):4851. [ Links ]
17. Rao CC, Krishna G. Anaesthetic considerations for magnetic resonance imaging. Ann Acad Med Singapore. 1994;23(4):5315. [ Links ]
18. Gozal D, Drengrer B, Levin PD, Kadari A, Gozal Y. A pediatric sedation/anesthesia program with dedicated care by anesthesiologists and nurses for procedures outside the operating room. J Pediatr. 2004;145(1):4752. [ Links ]
19. Fernández I, Galán C, Torre C. Comparación de ketaminamidazolam con propofolmidazolam para sedación y analgesia en pediatría. Bol Pediatr. 2000;40(171):1923. [ Links ]
20. Usher A, Kearney R, Tsui B. Propofol total intravenous anesthesia for MRI in children. Paediatr Anaesth. 2005;15(1):238. [ Links ]
21. Rashed AH, Shayevitz JR. Deep sedation wih propofol for children undergoing ambulatory magnetic resonance imaging of the brain: Experiencie from a pediatric intensive. Pediatric Critical Care Medicine. 2003;4(4):4548. [ Links ]











 texto en
texto en