Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.3 Bogotá jul./set. 2013
Endoscopic management of pancreatic pseudocysts
Martín Alonso Gómez Zuleta, MD. (1)
(1) Assistant Professor of Gastroenterology in the Department of Internal Medicine at the Universidad Nacional de Colombia. Gastroenterologist at Hospital El Tunal in Bogotá, Colombia.
Received: 03-10-12 Accepted: 26-06-13
Abstract
One of the complications of acute pancreatitis, which has a high prevalence in our environment, is the formation of pseudocysts. Some pseudocysts are spontaneously reabsorbed but others require intervention. Since interventions are often surgical, both morbidity and mortality rates are high. Radiological, endoscopic and other approaches have lower morbidity and mortality rates but are less well known in our country.
This is a study of our experience with 9 cases of endoscopic management of pseudocysts. We briefly present these cases, review the subject and provide a step by step description of a drainage method that is easily applicable by physicians interested in the subject.
Key words
Pseudocyst, acute pancreatitis, endoscopy, drainage.
Pancreatic pseudocysts are the most common late complication of acute pancreatitis, chronic pancreatitis, and pancreatic trauma. A pancreatic pseudocyst consists of an accumulation of amylase-rich pancreatic fluid caused by ductal disruption arising from limited pancreatic necrosis (1-3). It is diagnosed as a pseudocyst once it has persisted for more than 6weeks from the initial observation. Characteristically a pseudocyst is surrounded by a layer of fibrous granular tissue but has no real epithelium. This is the main difference with a true pancreatic cyst (4).
Symptoms depend on the location and extent of the collection of fluid. Symptoms include abdominal pain, early satiety, nausea, vomiting, duodenal obstruction, bile duct obstruction (jaundice), palpable mass, vascular occlusion, and fistulas (1, 5). This article reports our experience in the last year with 9 patients with pancreatic pseudocysts (table 1). Their average age was 40.1 years, the youngest was 8 years old, and the oldest was 78 years old. 55.5% of the patients were male. Most lesions were located in the pancreatic body and/or tail. They measured 119.3 mm on average. All lesions were resolved endoscopically, and there were no cases of recurrence in 11 months of follow-up. In 2cases the pseudocysts were drained with ERCP only, the other 7cases used endoscopy first followed by ERCP.
INDICATIONS FOR TREATMENT
Spontaneous resolution of a pancreatic pseudocyst occurs in only 40% of the cases and usually takes 3 to 6 months. Treatment is indicated for patients who present symptoms such as early satiety, abdominal pain, pyloric syndrome, jaundice, signs of infection or increased size of the pancreatic pseudocyst after 6 weeks (regardless of the initial size) (1, 2, 4). Indications in asymptomatic patients, less than 10% of all cases, are called "prophylactic" and include compression of large vessels by the pancreatic pseudocyst, hemorrhaging of the pseudocyst itself, and pleural pancreatic fistulas. Drainage is also indicated for patients with chronic pancreatitis and those with pancreatic stones since the spontaneous resolution rate of the pancreatic pseudocyst in these cases is very low (0-9%) (6).
DESCRIPTIONS OF CASES
Case 1
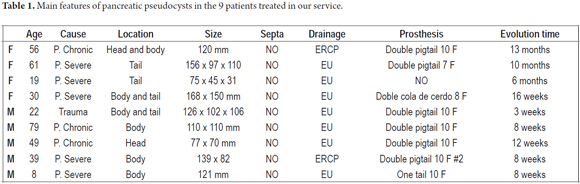
This 39 year old male patient had suffered 4 months of decreased appetite and feeling full after eating. The patient had an epigastric mass that gradually increased in size. Nine months earlier the patient had had biliary pancreatitis which required medical management. The patient was admitted to the emergency room with hematemesis. Upper endoscopy showed gastric extrinsic compression. An abdominal CT scan showed an image suggestive of a 139 x 82 mm pseudocyst without septa. Insertion of a double pigtail prosthesis was performed and patient recovered appropriately (Figure 1). 10 weeks later the prosthesis was removed. After one year of follow up there had been no recurrence.
Case 2
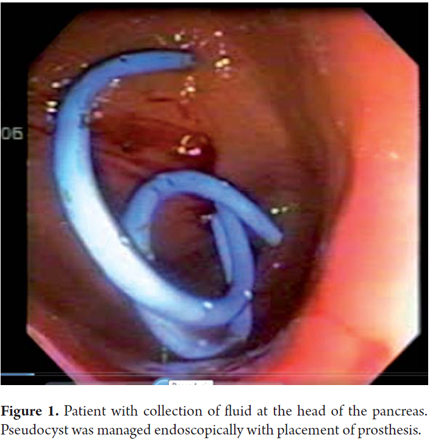
A 19 year old woman was admitted to the emergency room with epigastric abdominal pain and emesis. Six months earlier the patient had been discharged from the hospital following a laparoscopic cholecystectomy performed because of pancreatitis. An abdominal CT scan showed a 50 mm x 45 mm x 31mm collection of fluid in the body and tail of the pancreas. It was decided to drain the pancreas with a #19 needle. Patient evolved appropriately and was discharged one day after drainage. After one year of follow up there had been no recurrence (Figure 2).
Case 3
A 79 year old male patient was admitted to the emergency room with postprandial emesis, epigastric pain and gastric fullness. Patient had a history of acute biliary pancreatitis which had been treated with a cholecystectomy two months earlier. After admission to the emergency room an abdominal CT scan was performed. It showed an image compatible with a 110 mm x 110 mm cystic lesion (Figure 3). Transgastric endoscopic ultrasound (EU) guided drainage was performed through placement of a 10 Fr double pigtail prosthesis. Patiently evolved appropriately and was discharged a day later.
Case 4
A 49 year old patient with a history of pancreatic pseudocyst compromising the pancreatic head was admitted to the emergency room for epigastric pain. A CT scan showed a 77mm x 70mm collection of fluid in the pancreatic head. Transgastric drainage (Figure 4) was performed through placement of a 10 Fr double pigtail prosthesis. Patiently evolved appropriately and was discharged two days later. After one year of follow up there had been no recurrence.
Case 5
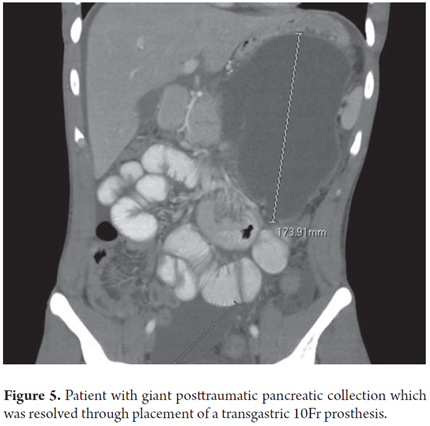
A 22 year old male patient who had been admitted to another institution after blunt abdominal trauma was admitted with an elevated amylase level. An abdominal CT scan showed a 126 mm x 102 mm x 106 mm collection of fluid in the pancreatic body and tail (Figure 5). Gastric endoscopy showed extrinsic compression. EU guided drainage was performed through placement of a 10 Fr double pigtail prosthesis. The clinical outcome has been excellent.
Case 6
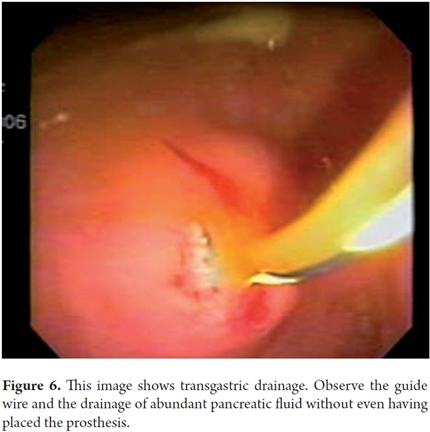
A 56 year old female patient who had undergone medical treatment for pancreatitis after a CT scan showed a 120 mm pancreatic pseudocyst, and who had improved and become asymptomatic, was admitted to the hospital after 11 months of outpatient follow-up when she presented symptoms and increased pseudocysts. Upper endoscopy showed no evidence of extrinsic gastric compression. Endoscopic ultrasound showed a 120mm diameter anechoic lesion in the pancreas which was compressing the pancreatic head and body. Transgastric drainage guided by fluoroscopy (Figure 6) was performed through placement of a 10 Fr double pigtail prosthesis. Patiently evolved appropriately and was discharged a day later. After 6 months of follow up there had been no recurrence.
Case 7
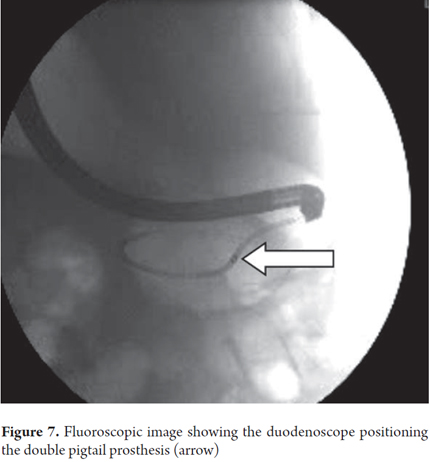
Eight months after a 61 year old female patient had been treated as an outpatient for severe acute pancreatitis and pancreatic pseudocyst formation, it was decided to hospitalize her for drainage because of the development of epigastric pain. An abdominal ultrasound showed a 156 mm x 97 mm x 110 mm collection of fluid. Transgastric endoscopic ultrasound (EU) guided drainage was performed through placement of a 7 Fr prosthesis (no other diameters were available). Patiently evolved appropriately and was discharged two days later (Figure 7).
Case 8
A 30 year old woman who was 10 weeks pregnant with more than one fetus was admitted after one month of acute pancreatitis. Patient had a progressively growing epigastric mass associated with epigastric pain and postprandial emesis. Abdominal ultrasound showed a 168 mm x 150 mm hypoechoic collection of fluid in the body and tail of the pancreas. Transgastric endoscopic ultrasound (EU) guided drainage was performed through placement of a double 8 Fr pigtail prostheses. At 26 weeks of her pregnancy the patient had a normal delivery. A CT scan taken 6 months later showed the prosthesis and no recurrence of the cyst (Figure 8).
Case 9
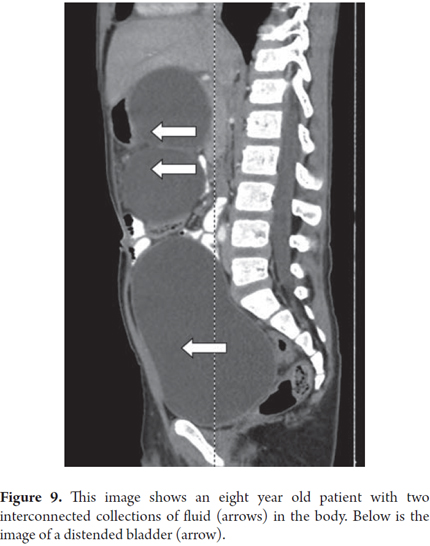
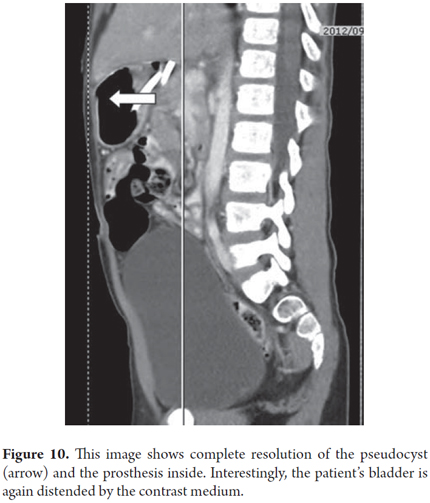
Two months after an eight year old child had spent three weeks in the intensive care unit with medical treatment for blunt abdominal trauma and severe acute pancreatitis, the patient continued to suffer from early satiety and abdominal pain. A CT scan showed two giant connected 121mm collections of fluid in the head and body of the pancreas (Figure 9). Transgastric endoscopic ultrasound (EU) guided drainage was performed through placement of a single 10 Fr pigtail prosthesis. The child's development was very good, and a follow-up CT scan showed no evidence of the lesion after 3 months (Figure 10). The prosthesis was removed. After 6 months of follow up there had been no recurrence and the patient continues to be asymptomatic.
DISCUSSION
Treatment options for patients with pancreatic pseudocysts include percutaneous drainage, surgery, and endoscopy. The latter's initial 90% success rate is the highest of the three, and 70% to 80% of these cases are resolved while the recurrence rate is between 10% and 15%. The recurrence rate could be lowered for cases of pancreatic necrosis in which the solid wastes interfere with proper drainage (6, 8, 9). There are two methods of endoscopic treatment: transmural drainage performed via the stomach or duodenum, and transpapillary drainage through the papilla. Combinations of the two methods are also possible. Drainage through the papilla has fewer complications but is less effective. It is only desirable for pseudocysts smaller than 5cm, and the pseudocyst must be connected with the pancreatic duct. This occurs in only about 40% of the cases (10). In our study this technique was not used.
Technical success is defined as the ability to insert at least an endostent between the pancreatic pseudocyst and the lumen of the gastrointestinal tract (stomach and duodenum) (10, 11), or as the resolution of the collection of fluid, although not necessarily of symptoms (12). Clinical success is defined as complete resolution of symptoms with a decrease in the size of the pseudocyst of at least 30% to 50% in the first month of treatment. All nine of our cases were 100% technically and clinically successful using the endoscopic method (11).
There are many studies that support the superiority of endoscopic treatment to surgery. One recent study that compared the two techniques showed that endoscopic treatment was superior to surgery in terms of cost, length of hospital stay, and quality of life (13). In another review that included 787 patients morbidity of the two methods was similar (13.3% vs. 16.0%, respectively), as was long-term recurrence of pseudocysts (10.7% vs. 9.8%, respectively), but endoscopic method resulted in a lower mortality rate (0.2% vs. 2.5%, respectively) (14).
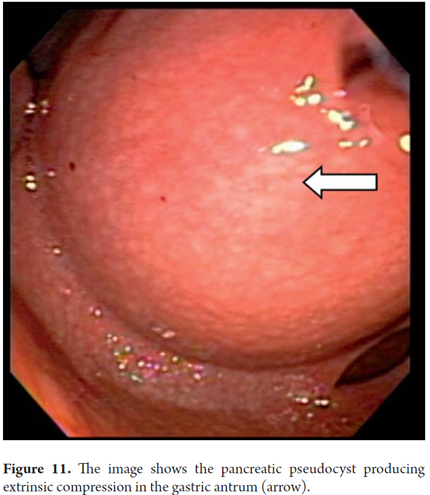
If the pseudocyst bulges or protrudes into the gastric cavity or duodenum, it is usually considered that it can be drained without the guidance of ultrasound endoscopy which is reserved only for cases where there is no compression of the lumen. Nevertheless, our group considers that all pancreatic pseudocysts should be drained with prior EU since there is always the risk of having a vessel in the gastric wall that separates the collection of fluid. These lesions are very dynamic and are very likely to break in the course of their evolution. This can be detected by EU. This was the case when a 9 year old girl was referred to us for drainage of a pseudocyst. An MRI (Figure 11) that had been taken eight days before indicted the pseudocyst. Nevertheless, when we evaluated the patient for drainage, we observed that it had broken into the cavity resulting in pancreatic ascites. In addition, it should be noted that for drainage of small pancreatic pseudocysts (5 cm), a #19 needle guided by EU is often sufficient for complete aspiration. If we choose the endoscopic route, transduodenal drainage is preferable to gastric drainage if both are feasible. Also, insertion of a dual 10 F pigtail prosthesis has fewer complications than insertion of a straight, and is therefore preferable. This is also the recommendation of the American Society for Gastrointestinal Endoscopy (ASGE). If it is possible, you should place two prostheses because the use of a double prosthesis has fewer recurrences than the use of only one. A follow up CT scan should be done 2 months after the procedure. If there is no evidence of collected fluid, the pseudocyst should be removed endoscopically. In our series, most patients' pseudocysts resolved within 2 or 3 months and only one patient required two prostheses.
A review of seven studies that evaluated 121 patients to compare cystoduodenostomies (transduodenal) with cystogastrostomies (transgastric) showed that long-term success was higher with the transduodenal approach than with the transgastric approach (59/71 [83.1%] vs. 32/50 [64.0%], p=0.019). Morbidity rates were identical (10%) (15). This may be related the fact that of cystoduodenal fistulas are more permeable than cystogastric fistulas (16-17). Nevertheless, in our experience it is more difficult to address the duodenum than the gastric cavity given the difficulty of positioning the equipment.
What is our technique?
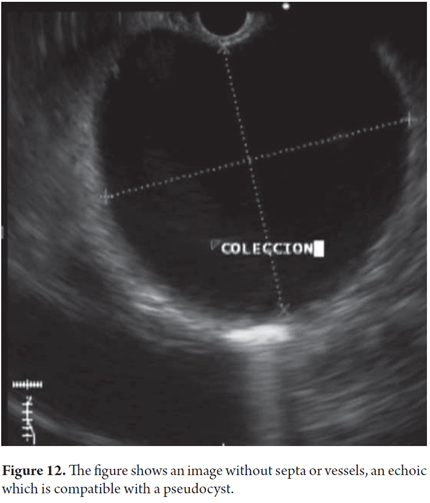
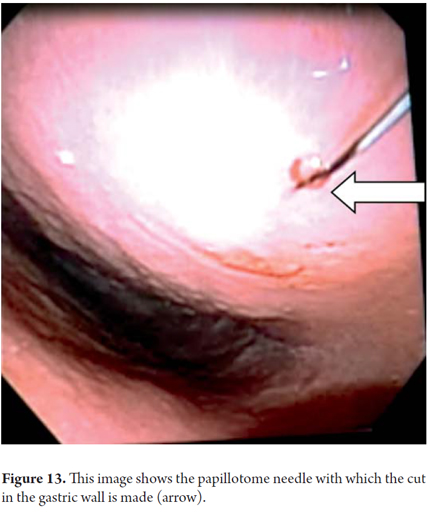
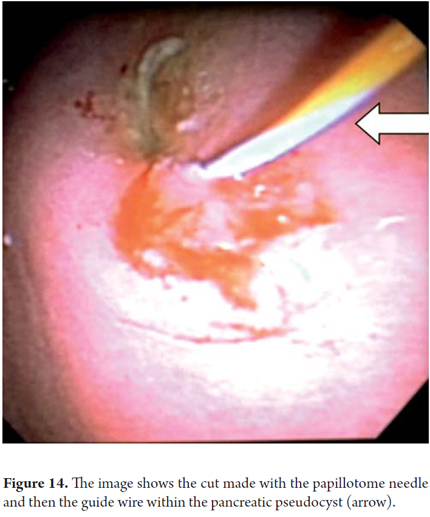
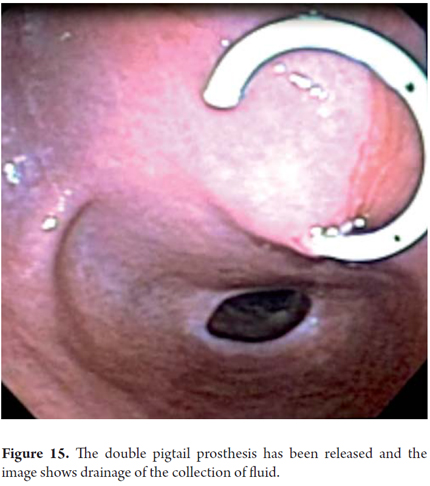
We will describe the technique we have been using in our service for about5 years. The patient is sedated by an anesthesiologist and is placed in left lateral position. Then we introduce the endoscope. Next, we find the pseudocyst (Figure 11) and the wall that separates it from the gastric lumen. We evaluate it by measuring its thickness and using a Doppler signal to rule out the presence of vessels (Figure 12). Once the site chosen has been located, we mark the area with biopsy forceps (or ink), then we remove the echoendoscope and replace it with a duodenoscope which we believe provides better angulation for drainage. After introducing a guided papillotome needle with coagulation current set at 30V, the wall is perforated (Figure 13). Once entry to the pancreatic pseudocyst has been achieved, the guide wire should be advanced guided by fluoroscopy (this is not strictly necessary: we have drained some cases in the endoscopy room). The coagulation hole can now be extended to 2 or 3 mm in diameter using the same papillotome for cutting (Figure 14). This will allow passage of the prosthesis (this step can be done with a biliary dilatation balloon, but it is more expensive). The papillotome is then removed and the prosthesis is advanced (Figure 15). Then the orifice is cannulated again, and - using the same method - we place the other prosthesis. Double pigtail 10 Fr type prostheses should be placed. For 5days following the procedure, 1.5 grams of ampicillin/sulbactam should be administered orally every 6 hours, and 1 tablet of fluconazole should be administered daily (8, 9). Because of the high rates of recurrence, prostheses should remain in situ for 8 weeks. At the point another CT scan should be performed. If there are no residual lesions, the prostheses should be removed (4).
Complications can arise during the procedure or insertion of the prostheses. Bleeding, one of the most feared complications, may require sclerotherapy or surgery.Complications of transpapillary drainage are related to performance of ERCPs. They include pancreatitis, bacteremia and the formation of abcesses. The primary complications related to prostheses are occlusion and migration (9).
In conclusion, endoscopic therapy for drainage of pancreatic pseudocysts is a safe, effective and minimally invasive procedure which can even be performed on an outpatient basis. In our series there were no complications or recurrences in the follow-up period, so we believe that this is the procedure of choice for management of these patients. Surgery should be reserved only for cases in which endoscopy fails and for cases for which this procedure is contraindicated.
REFERENCES
1. Douglas A Howell, Raj J Shah, Christopher Lawrence. Diagnosis and management of pseudocysts of the pancreas, Uptodate, junio 2011. [ Links ]
2. Todd H. Baron. Treatment of Pancreatic Pseudocysts, Pancreatic Necrosis, and Pancreatic Duct Leaks. Gastrointest Endoscopy Clin N Am 2007; 17: 559-579. [ Links ]
3. Steven D Freedman. Complications of chronic pancreatitis, Uptodate, Nov 2008. [ Links ]
4. Cheruvu CV, Clarke MG, Prentice M, Eyre-Brook IA. Conservative treatment as an option in the management of pancreatic pseudocyst. Ann R Coll Surg Engl 2003; 85: 313. [ Links ]
5. Ignacio Esquivel Ledesma. Drenaje abierto de pseudoquiste pancreático. Revista de Especialidades Médico-Quirúrgicas 2011; 16(4): 256-259. [ Links ]
6. Douglas A Howell, Raj J Shah. Endoscopic management of pseudocysts of the pancreas: Efficacy and complications, Uptodate Oct 2010. [ Links ]
7. William H. Nealon, Eric Walser. Main Pancreatic Ductal Anatomy Can Direct Choice of Modality for Treating Pancreatic Pseudocysts (Surgery Versus Percutaneous Drainage). Annals of surgery 2008; 235: 751-758. [ Links ]
8. Güitrón-Cantú A, Adalid-Martínez R, Gutiérrez-Bermúdez J. Drenaje de seudoquistes pancreáticos por vía transpapilar o transmural, Rev Gastroenterol Mex 2005; 5: 38-45. [ Links ]
9. Aghadassi A, Mayerle J, Kraft M, Sielenkämper A. Pancreatic pseudocysts-when and how to treat? HPB (Oxford) 2006; 8: 432-441. [ Links ]
10. Cahen D, Rauws E, Fockens P, et al. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy 2005; 37: 977–983. [ Links ]
11. Park DH, Lee SS, Moon SH, et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy 2009; 41: 842-848. [ Links ]
12. Hookey LC, Debroux S, Delhaye M, et al. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc 2006; 63: 635-643. [ Links ]
13. Varadarajulu S, Trevino J, Wilcox CM, et al. Randomized trial comparing EUS and surgery for pancreatic pseudocyst drainage. Gastrointest Endosc 2010; 71: AB116-AB116. [ Links ]
14. Rosso E, Alexakis N, Ghaneh P et al. Pancreatic pseudocyst in chronic pancreatitis: endoscopic and surgical treatment. Dig Surg 2003; 20: 397-406. [ Links ]
15. Beckingham IJ, Krige JE, Bornman PC, et al. Endoscopic management of pancreatic pseudocysts. Br J Surg 1997; 84: 1638-1645. [ Links ]
16. Funnell IC, Bornman PC, Krige JE, et al. Endoscopic drainage of traumatic pancreatic pseudocyst. Br J Surg 1994; 81: 879-881. [ Links ]
17. Cremer M, Deviere J, Engelholm L. Endoscopic management of cysts and pseudocysts in chronic pancreatitis: long-term follow-up after 7 years of experience. Gastrointest Endosc 1989; 35: 1-9. [ Links ]











 texto en
texto en