Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
CT&F - Ciencia, Tecnología y Futuro
versión impresa ISSN 0122-5383versión On-line ISSN 2382-4581
C.T.F Cienc. Tecnol. Futuro v.3 n.4 Bucaramanga ene./dic. 2008
1Ecopetrol S.A -Instituto Colombiano del Petróleo, A.A. 4185 Bucaramanga, Santander Colombia
2Universidad Industrial de Santander (UIS), Escuela de Ingenieria Química, Bucaramanga, Santander, Colombia
e-mail: adan.leon@ecopetrol.com.co yovani_96@hotmail.com
(Received April 30, 2008; Accepted Nov. 14, 2008)
* To whom correspondence may be addressed
ABSTRACT
Knowledge of critical properties and the acentric factor is required in phase-equilibrium studies in different extraction processes conducted in the petroleum industry, particularly in the solvent deasphalting process. Correlations to estimate critical temperature, critical pressure and acentric factor values of SARA (Saturated, Aromatic, Resin, and Asphaltene) fractions of vacuum residue from the Barrancabermeja Refinery were determined from their physical properties such as density (molar volume) and molecular weight. New correlations for critical property prediction were evaluated using model molecules and the Avaullee and Satou's group contribution methods, respectively.
Key words: vacuum residue, petroleum fraction, critical property, refinería de Barrancabermeja. Ciencia, Tecnología y Futuro.
RESUMEN
En los estudios de equilibrio de fases, de los diferentes procesos de extracción en la industria del petróleo y en particular en el proceso de desasfaltado con solventes, se requiere el conocimiento de las propiedades críticas y factor acéntrico. Por esta razón, en el presente trabajo se determinaron correlaciones para estimar temperatura crítica, presión crítica y factor acéntrico de las fracciones SARA (Saturados, Aromáticos, Resinas y Asfaltenos) de fondos de vacío provenientes de la Refinería de Barrancabermeja, a partir de sus propiedades físicas como densidad (volumen molar) y peso molecular. Las nuevas correlaciones para la predicción de propiedades críticas fueron evaluadas usando moléculas modelo, mediante los métodos de contribución de grupos de Avaullee y Satou, respectivamente.
Palabras Clave: Fondos de Vacio, fracciones de petróleo, propiedades críticas, refinería de Barrancabermeja.
INTRODUCTION
Vacuum residue can be separated into four distinctive fractions named saturated, aromatic, resin and asphaltene fractions. These, in turn, contain Ni and V as well as heteroatoms such as S, N, and O (Andersen & Birdi, 1990; Suoqi Zhao, Renan Wang, & Shixioing Lin 2006). Metals and heteroatoms are considered as polluting agents promoting corrosion and catalyst poisoning at the Hydrotreatment (HDT) and catalytic Craking (FCC) units. Vacuum residue undertake the deasphalting process with propane-butane solvent in order to prepare loads for HDT aiming at the decrease of pollutant compounds. At present, several works on the deasphalting process are being developed. These works are based on a study oriented toward the design of thermodynamic mathematical models to interpret the phase-equilibrium behavior as it is explained by Suoqi Zhao et al. (2006), Parra and Cañas (2007), and León (2008).
Thermodynamic studies based on state equations require to know the critical temperature, critical pressure, and acentric factor values of both the fractions obtained by SARA separation analysis of vacuum residue and the deasphalting process. Important correlations are observed in the petroleum industry regarding the study of physicochemical and critical properties of non-polar compounds and heavy crude oil fractions. A detailed explanation is published by Jalowka and Daubert (1986), Riazi and Al-Sahhaf (1987), Zhang Jianzhong et al. (1998), among other authors.
Much effort has been invested so far to find the appropriate correlations in the prediction of critical and physicochemical properties of vacuum residue. The Group Contribution Method has demonstrated to be one of the most competitive and effective methods. This method divides molecules in independent functional groups being this classification, to some extent, arbitrary. The molecule - molecule interactions correspond to the weighed sum of interactions among their corresponding functional groups. It is possible to determine certain properties of other molecular structures, even though no experimental data are available (Prausnitz, 2000). Calculation is based on this method, and on the study of experimental data, similarities between the structure and the physical properties of compounds.
In their study of pure compounds and their isomers, Constantinou and Gani (1994) determined the appropriate parameters to detect properties such as: boiling point, critical temperature, critical pressure, critical volume, vaporization standard enthalpy, standard Gibbs energy and formation enthalpy. Correlation of critical properties in heavy crude oils and bitumens, as well as of hydrocarbon and liquid carbon fractions were developed in similar works by Cheng-Tze Fu and Rao Puttagunta (1986); and Zhang Jianzhong, Zhan Biao, and Zhao Suoqui (1998), respectively.
Despite the chemical complexity of vacuum residue SARA fractions, and scarce, even lacking of information about their properties to facilitate the calculation, several authors have considered that the fractions mentioned above are composed by polycondensed aromatic species. These are represented by model molecules. Their critical and physicochemical properties can be determined in function of their molecular weight and density (Garnier, Neau, Alessi, Cortesi, and Kikic, 1999; Akbarzadeh, Alboudwarej, Ayatollah, & Yarranton, 2004); Alboudwarej, Akbarzadeh, Beck, and Yarranton (2003); Golam, and Mansouri (2006). For the prediction of heavy crude oil mixture densities, bitumens, and their SARA fractions, parameters of the state equations used were adjusted from estimated correlations of critical temperature, critical pressure, and acentric factor for each fraction based on model molecules such as naphthalene, antracene, phenantrene, pyrene, and perilene proposed by Garnier et al. (1999) and evaluated with the Group Contribution Method proponed by Avaullee et al. (1997).
Murgich, Rodríguez, and Aray (1995) and other authors such as Zander et al. (1987), Strausz, Mojelsky, and Lown (1992); Kotlyar, Woods, and Sparks (2001); Rogel, León, Espidel and González (1999); Rogel (2000); Rogel and Carbognani (2003); Wakeham, Cholakov and Stateva (2003), have utilized molecular simulation to provide representative structures of SARA fraction molecules in different types of hydrocarbon compounds, based on descriptive parameters and experimental data.
A set of model molecules of greater molecular weight was selected in this study. Their critical properties and density (molar volume) were evaluated with the methods proposed by Avaullee, Trrasy, Neau, and Jaubert (1997); Satou, Nakamura, Chiba, and Hattori (2000), in order to find a tendency to predict typical Colombian vacuum residue SARA fraction properties in a more accurate manner.
EXPERIMENTAL METHODOLOGY
Nine vacuum residue from vacuum distillation units at the Barrancabermeja Refinery were selected. The work was conducted in two stages: first, the SARA separation analysis was used in the vacuum residue. The molecular weight and density values were determined for each fraction found. Secondly, based on the study of model molecules, appropriate correlations were developed in order to determine critical temperature, critical pressure, and acentric value for SARA fractions based on their molecular weight and density. The corresponding tests were conducted at Instituto Colombiano del Petróleo (ICP), Ecopetrol S.A.
SARA Separation analysis
Liquid chromatography is preceded by an asphaltene precipitation process. A ratio of 1:40 volume of vacuum residue and n-heptane was used, thus conforming to the ASTM D-4124 (1997) regulation. The separation of the maltene fraction was validated based on the ASTM-D 2007 (1998) regulation. The saturated carbon elution was conducted with n-heptane. Aromatic hydrocarbons were treated by a reflux extraction process, with a mixture toluene-ketone 1:1 volume in a silicon column. The resin fraction was then obtained by reflux extraction with a toluene-ketone mixture 1:1 in a clay column. A liquid chromatography separation method has been used in this study at preparative scale in order to obtain significant amounts of each SARA fraction.
Density
The test was conducted in each selected vacuum residue and its SARA fractions. Toluene was selected as the preferential solvent according to the ASTM D 2320 (1998) regulation. Three solutions with concentrations between 0 ppm and 15000 ppm were prepared during each fraction analysis. The real density of each sample was determined by linear regression, extrapolating solute concentration at 100%. A DMA 48 digital densimeter was used as it is recommended in the standard procedure set out by the ASTM D-4052 regulation. Tests were run at 20°C, 40°C and 70°C (291,15 K; 313,15 K; 343,15 K).
Molecular weight
The distribution profile of samples in heavy crude oils, atmospheric residues, and vacuum residues is obtained from the high temperature simulation distillation analysis. Results of this study show the proportion of the sample weight percentage in distilled material according to the temperature used. However, the average molecular weight of vacuum residue and their SAR (Saturated, Aromatic, and Resin) fractions was determined from the distillation curve and the composition percentage of the standard sample. The measurement of the asphaltene fraction was determined by subtracting the molecular weight of SAR fractions from the molecular weight of the vacuum residue. The calibration sample is normal paraffin sample ranging from n-C5 to n-C100. The solvent used in the in the test is CS2. The analysis was conducted at the HP 6890 gas chromatograph Ander the specifications set out by the ASTM D-6352 regulation.
RESULTS
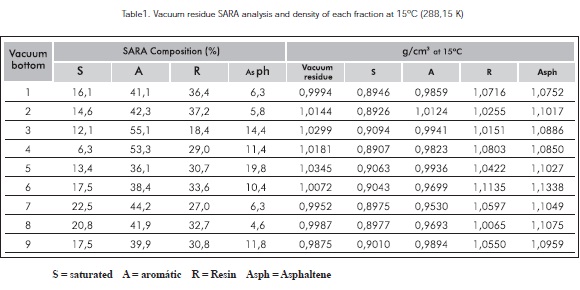
Table 1 shows the results obtained by the SARA of the vacuum residue selected and their corresponding density at 15°C (288,15 K).
The following density correlations for each vacuum residue SARA fraction in function of temperatura have been found in the density studies at 20°C, 40°C and 70°C (293,15 K; 313,15 K y 343,15 K) for each SARA fraction:
- Saturated fraction
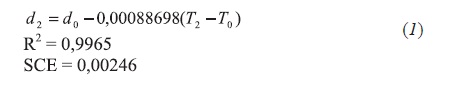
- Aromatic fraction
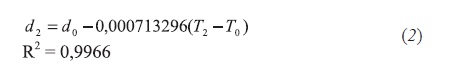
- Resin fraction
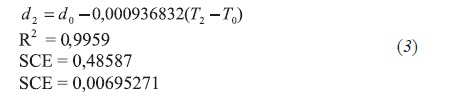
- Asphaltene fraction

Molecular weight
The values found for each SARA fraction vary according to the analytical method used. Akbarzadeh et al. (2003) consider that the molecular weight is related to the aggregation average number and that this number depends on the composition, concentration, and temperature. However, a component distribution method by simulated distillation has been used in this work, based on paraffin molecules as standard molecules. Furthermore, the gamma distribution function has been considered to calculate the average molecular weight of vacuum residue and their SAR fractions.
The gamma distribution has been used as a mathematical function to characterize properties of hydrocarbon mixtures (Behrenbruch et al., 2007). The probability distribution function for a twoparameter.
Gamma distribution conforms to Equation 5:

Where,
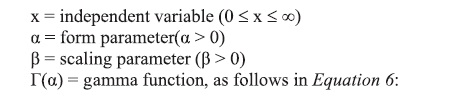

The cumulative probability function is expressed as Equation 7:

The α and β parameters described in the expressions above are estimated to obtain a better tendency of the above mentioned data regarding experimental data.
The two-parameter gamma distribution model can be transformed into a three-parameter gamma distribution model, as it has been analyzed by Whitson (Curtis, 2000; & Riazi, 2005). Their studies report that this model is appropriate to describe the molecular weight distribution in hydrocarbon mixture of different kinds. In terms of molecular weight, the gamma probability distribution function is the following Equation 8:
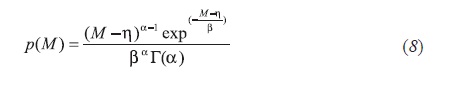
However, the β and η parameters can be apporoximately expressed in funtion of the α parameter as follows Equation 9:

and,
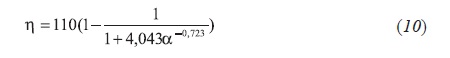
Where:

Mi and xi correspond to the molecular weight and the molar fraction of each component found in the calibration curve.
The α values of the vacuum residue and their corresponding SAR fractions are adjusted according to the determination coefficient expression Equation 12:
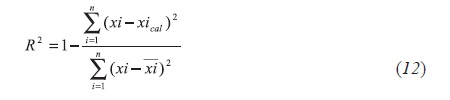
Where,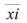 , and xical are the experimental, average, and calculated molar fractions, respectively, of the pseudocompounds i found in each distribution curve.
, and xical are the experimental, average, and calculated molar fractions, respectively, of the pseudocompounds i found in each distribution curve.
Besides, considering that the percentage weight of recovery is not total when applying the simulation distillation technique for the fractions in this study, components with molecular weights greater than C100 (Mhi > C100) were assumed from the study of model molecules in order to obtain a better representation of each sample distribution. The molar fractions of components greater than C100 (hi) are adjusted in a parallel manner regarding the α and β parameters until an approximate R2 of 1 was obtained (Figure 1).
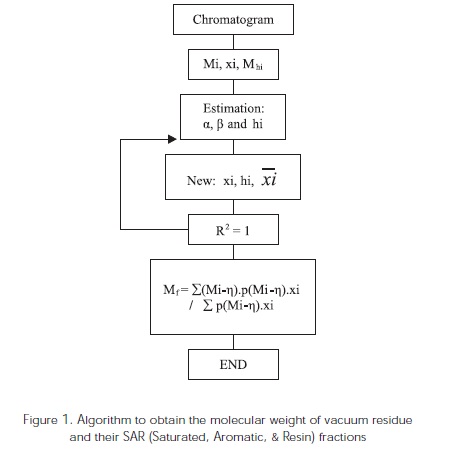
Both the vacuum residue and their SARA fractions are composed by a mixture of high-molecular weight components so as the carbon number increases in the structures belonging to different hydrocarbon families, the difficulty for describing the different fractions also increases.
As an example, Figure 2 shows data distribution obtained from simulated distillation of the vaccum residue # 6 and its corresponding SAR fractions. The saturated and aromatic fractions exhibit a better distribution trend between experimental data and the data established with the gamma distribution function due mainly to the chemical composition of these fractions.
Zander et al. (1987); Strausz, Mojelsky, and Lown (1992); Kotlyar et al. (2001); Rogel et al. (1999, 2000 and 2003) and Wakeham et al. (2003), have demon strated by analytic simulation and techniques that the compounds in the saturated fraction consist of linear chains, ramified chains and cycloparaffins. Furthermore, it has been considered that the aromatic fraction is largely composed by a aromatic cores with condensed sets of naphtenic rings with several lateral alkyl chains of different length. In general terms, the chemical nature of the two fractions mentioned above and their low molecular weights exhibit favorable effects with the simulated distillation analysis regarding component distribution.
Resin fraction is found in average proportion in the vacuum residue (Table 1). Its chemical nature makes it sensible to temperature changees (Barth et al.,1984. & Gawel et al., 1987). Furthermore, this fraction contains several rings in its structure that are condensed with polar groups such as O and S. These rings make inter-molecular bonds and bridges thus helping in the synthesis of high-molecular weight strucutures (Poirier & Sawatzky, 1990).
Due to the simulated distillation test conditions at high temperature, the vacuum residue and resins have more important deviations as a result of the synthesis of high-molecular weight molecules and coke (Wang, & Anthony, 2003). On the other hand, it is considered that the interaction among the compounds of the different fractions is greater, thus avoiding the easy elution of components. As temperature increases, the formation of condensed aromatic rings increases in the vacuum bottom and resin fraction. This decreases the distillation yield. Furthermore, the polarization ability of asphaltenes due to their aromatic nature is helpful in the creation of strong attraction to similar particles, especially high molecular weight resins. The vacuum residue show better distribution in comparison to the resin fraction. This influence has to be taken into account due to the saturated and aromatic fraction proportion.
Even though the percentage of distilled compounds or recovery using this technique is lower for vacuum residue and resins compared to the saturated and aromatic fraction, with the gamma distribution functions permitted values have been established within the intervals found, as it is mentioned by Murgich et al. (1995), Kotlyar et al. (2001), Akbarzadeh et al. (2004) and Golam, and Mansouri (2006), among other authors.
Table 2 reports the molecular weights of vacuum residue and their SARA fractions. Despite of the results established, it is not possible now to compare it to other technique, although its principles are totally different.
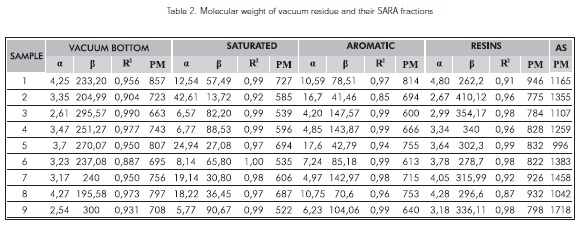
An average is obtained with the analysis of simulated distribution. It shows the distribution of a wide variety of constituent molecule families of vacuum residuefractions that is appropriate for the presentation of a molecular weight range.
Critical properties
Vacuum residue SARA fractions do not present a unique single and exact correlation to estimate thier physical and critical properties. Therefore, in order to facilitate calculation, it has been established that this fractions are constituted by molecule species or types.
Two critical property correlation types have been established from the molecules reported in this work in function of the molecular weight and the molecular weight-density for the vacuum bottom SARA fractions.
Table 3. shows the critical properties of model molecules evaluated with the Group Contribution Method proponed by Avaullee et al. (1997) and Satou et al. (2000).
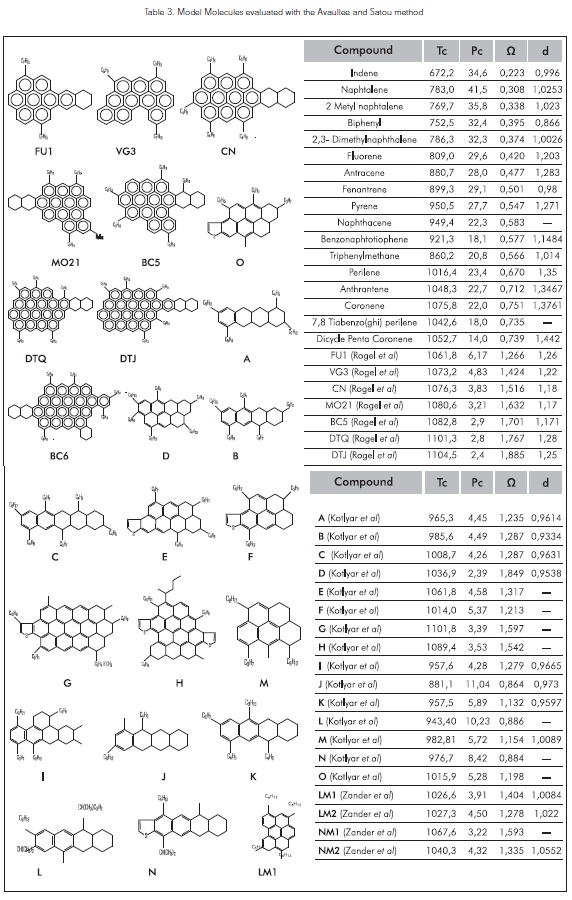
Critical properties in function of the molecular weight
Based on the work conducted by Akbarzadeh et al. (2003), Alboudwarej et al. (2004); Golam, R., and Mansouri. (2006); this work considers molecules of greater molecular weight in order to find a better predictive correlation tendency regarding the critical properties of vacuum residue SARA fractions (Figure 3).
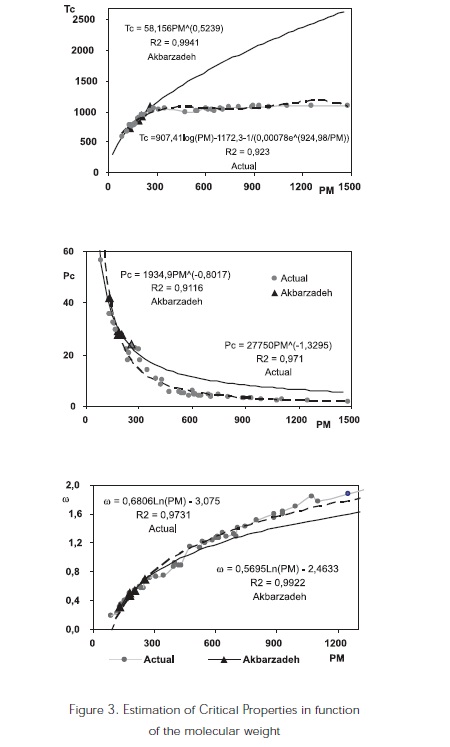
Table 4 evaluates the properties of eight compounds to compare the correlations found in this study (preseent correlations) to the values reported by Akbarzadeh et al., 2003
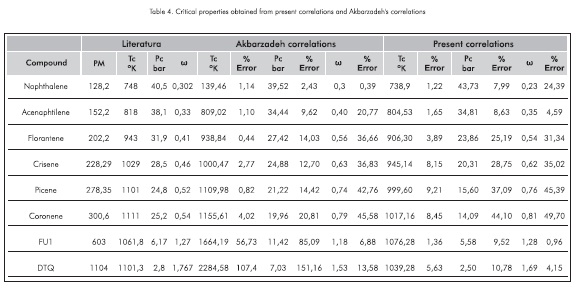
Base don the results found, present correlations exhibit a good tendency for high-molecular weight molecules.
If the molecular weights found for the vacuum residue SARA fractions are considered, the best correlations to evaluate their critical properties are the present correlations.
Critical properties in function of molecular weight and density
As critical properties, density data for hydrocarbons and petroleum fractions can be obtained from adjusted correlations, from estimated correlations by several authors such as: Kesler-Lee, Cavett, Riazi - Daubert, and Nokay (Riazi, 1987). However, prediction of new correlations of both physical and critical properties for petroleum fractions can be determined from two physical properties as parameters suitable for the characterization of molecular forces and size of a given compound. Therefore, new correlations were developed based on the expressions reported by Zhang Jianzhong et al. (1998), Equations 13 and 14:

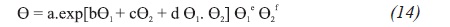
Where:
Θ = Correlation of the adjusted critical property
Θ1, Θ2 = Evaluated physical properties
By applying the above mentioned correlations for vacuum bottom SARA fractions, the following critical property correlations were found in function of density and molecular weight:


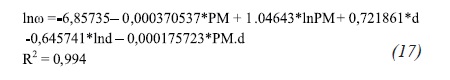
Table 5 shows the properties of the evaluated compounds with the correlations found (Equations 15 through 17).
Comparing the results of the Table 4 to Table 5, the critical properties related to molecular weight and density have the least error percentages. This percentage value is even lower for compounds with high molecular weight. In addition, these latter correlations adjust even more for a greater and more number of components.
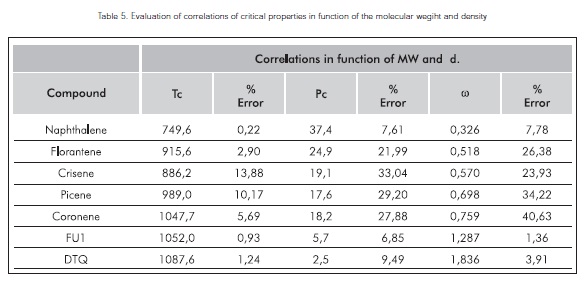
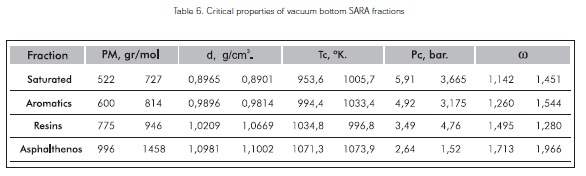
Table 6 reports the interval of specific critical properties of these vacuum residue SARA fractions considering the correlation of the critical propertiesfound, the density properties at 20°C (293,15 K) and the molecular weight of saturated, aromatic, resin, and asphalthene fractions.
Comparing the results in Table 6 to some similar hydrocarbon works, (Akbarzadeh et al., 2003, Alboudwarej et al., 2004, & Golam et al., 2006), it is possible to conclude that the methodology developed is appropriate for the estimation of properties such as critical temperature, critical pressure, and acentric factor of vacuum residue SARA fractions.
CONCLUSIONS
-
The gamma distribution probability function is a suitable tool for predicting of molecular weight in vacuum residue and their saturated, aromatic, and resin fractions.
-
The correlation of critical properties and acentric factor has been found in function of density and molecular weight for typically Colombian vacuum residue SARA fractions. This is achieved through the study of model molecules and selected group contribution method.
-
The estimated correlations for critical properties in function of density and molecular weight can be adjusted for a broad range of condensed aromatic compounds.
ACKNOWLEGMENTS
The authors express their gratitude to Instituto Colombiano del Petróleo (ICP), Ecopetrol S.A. and to Universidad Industrial de Santander (UIS) for their financial, scientific and human support in the design and development of this work.
REFERENCES
Akbarzadeh, K., Alboudwarej, H., Ayatollahi, S., &Yarranton, W. (2004). Estimation of SARA fraction properties with the SRK EOS. JCPT, 43 (9), 31-39. [ Links ]
Akbarzadeh, K., Alboudwarej, H., Beck, J., & Yarranton, H. (2003). A generalized regular solution model for asphaltene precipitation from bitumens and solvents. AICHE Journal, 49 (11), 2948-2956. [ Links ]
Alboudwarej, H., Akbarzadeh, K., Beck, J., & Yarranton, W. H. (2003). Regular solution model for asphaltene precipitation from bitumens and solvents. AICHE Journal, 49 (11), 2948-2956. [ Links ]
ASTM D-4052 (1996). American Society for Testing and Materials. Standard Test Method for Density and Relative Density of Liquids by Digital Density Meter. Philadelphia. [ Links ]
ASTM D-4124 (1997). American Society for Testing and Materials. Standard test methods for separation of asphalt into four fractions. Philadelphia. [ Links ]
ASTM D (2007). American Society for Testing and Materials. Standard test method for characteristic groups in rubber extender and processing oils and other petroleum-derived oils by the clay-gel absorption chromatographic method. Philadelphia. [ Links ]
ASTM D -2320 (1998). American Society for Testing and Materials. Standard test method for density (relative density) of solid pitch (pycnometer method). Philadelfhia. [ Links ]
ASTM D-6352. American Society for Testing and Materials. Método de ensayo para la distribución de puntos de ebullición de destilados de petróleo en un rango de ebullición de 174 a 700°C por cromatografía de gases. [ Links ]
Andersen, S., & Birdi, K. (1990). Influence of temperature and solvent on the precipitation of asphaltenes. Fuel Science and Technology International, 8 (6), 593-615. [ Links ]
Avaullee, L., Trrasy, L., Neau, E., & Jaubert, J. N. (1997). Thermodynamic modelling for petroleum fluids: I. Equation of state and group contribution for the estimation of thermodynamic parameters of heavy hydrocarbons. Fluid Phase Equilibria,139: 155-170. [ Links ]
Barth, E. J. (1984). Asphalt: Science and Tecnology. Gordon and Breach Science,112-170 and 182-207. [ Links ]
Behrenbruch, P., & Dedigama, T. (2007). Clasification and characterization of crude oils based on distillation properties. J. Petroleum Scien. and Engineer., 57: 166-170. [ Links ]
Cheng-Tze Fu., & Rao Puttagunta. (1986). Pseudo-critical properties of heavy oils and bitumens. Fluid Phase Equilibria, 30: 281-295. [ Links ]
Constantinou, L., & Gani, R. (1994). New Group Contribution Method for Estimating Properties of Pure Compounds. AICHE Journal, 40 (10), 1697-1710. [ Links ]
Curtis-H., Whitson, & Riazi, M. (2000). Phase behavior. Monograph SPE Society of Petroleum Engineers. 20: 67-87 and 120-195. [ Links ]
Garnier, S,. Neau, E., Alessi, P., Cortesi, A., & Kikic, I. (1999). Modelling solubility of solids in supercritical fluids using fusion properties. Fluid Phase Equilibria, 158-160: 491-500. [ Links ]
Gawel, I. (1987). Structural Investigation of Asphalts produced from paraffinic-base crude oil by different methods. Fuel, 66 (5), 618-621. [ Links ]
Golam, R., & Mansouri, S. (2006). Cubic EOS calculates heavy oil SARA fractions. Oil and Gas Journal, Dec., 11, 48-49. [ Links ]
Jalowka, J., & Daubert, T. (1986). Group contribution method to predict critical temperature and pressure of hydrocarbons. Ind. Eng. Chem. Process. Des. Dev. (25), 139-142. [ Links ]
Kotlyar, L. S.,Woods, R. J., & Sparks, B. D. (2001). Effect of thermal and hydro-catalytic treatment on the molecular chemistry of narrow fractions of athabasca bitumen pitch. Energy and Fuel, 15: 113-119. [ Links ]
León-B. Adan. (2008). Modelamiento Termodinámico del desasfaltado de fondos de vacío basado en la ecuación de estado de Peng - Robinson y la técnica de quimiometría. Tesis de maestría en Ingenierìa Química,Universidad Industrial de Santander, UIS. [ Links ]
Murgich, J., Rodríguez, M., & Aray, Y. (1995). Molecular recognition and molecular mechanics of micelles of some model Asphaltenes and Resins. Centro de Química, IVIC, Apartado 21827, Caracas 1020A, Venezuela. [ Links ]
Parra, J., Martha., & Cañas, A., Wilson. (2007). Thermodynamic model for solvent deasphalting of vacuum residue. Proceedings annual meeting, AICHEJ Journal Nov. 4. [ Links ]
Poirier, M. A., & Sawatzky, H. (1990). Changes in Chemical component type composition and effect on rheological properties of asphalts, preprints. Symposium on Chemistry and Characterization of Asphalts, Div. Pet. Chem., Am. Chem. Soc., 35 (3), 301-307. [ Links ]
Prausnitz, J. (2000). Molecular Thermodynamics of Fluid- Phase Equilibria. University of California, Prentice Hall PTR (Oct. 22 1988). [ Links ]
Riazi, M. R., & Al-Sahhaf, T., (1996). Physical properties of heavy petroleum fractions and crude oils. Fluid Phase Equilibria,117: 217-224. [ Links ]
Riazi, M., & Daubert, T. (1987). Characterization parameters for petroleum fractions. Ind. Eng. Chem. Res., 26: 755-759. [ Links ]
Riazi, M. R. (2005). Characterization and properties of petroleum fractions. ASTM, Printed in Philadelphia, 150-197. [ Links ]
Rogel, E., & Carbognani, L. (2003). Density estimation of asphaltenes using molecular dynamics simulations. Energy and Fuels, 17: 378-386. [ Links ]
Rogel, E. (2000). Simulation of interactions in asphaltene aggregates. Departamento de Producción, PDVSAINTEVEP, Apartado. 76343, Caracas-1070A, Venezuela. Revised Manuscript Received. [ Links ]
Rogel, E., Leon, O., Espidel, J., & González, J. (1999). SPE Latin American and Caribbean Petroleum Engineering Conference, SPE53998, Venezuela. [ Links ]
Satou, M., Nakamura, T., Chiba, T., & Hattori, H. (2000). Contributions of aromatic conjunction and aromatic inner carbons to molar volume of polyaromatic hydrocarbons. Fuel, 79: 1057-1066. [ Links ]
Strausz, O. P., Mojelsky, T. W., & Lown, E. M. (1992) Fuel, The molecular structure of asphaltene: an unfolding story. 7: 1355-1362. [ Links ]
Suoqi Zhao, Renan Wang, & Shixioing Lin. (2006). High - pressure phase behavior and equilibria for Chinese Petroleum Residua and Light Hydrocarbon Systems. Part I. Petroleum Science and Technology, 24: 285-295. [ Links ]
Suoqi Zhao, Renan Wang, & Shixioing Lin. (2006). High -pressure phase behavior and equilibria for Chinese petroleum Residua and light hydrocarbon systems. Part II. Petroleum Science and Technology, 24: 297-318. [ Links ]
Wakeham, W. A., Cholakov, G., & Stateva, R. P. (2003). Liquid density and critical properties of hydrocarbons estimated from molecular structure. J. Amer. Chem. Soc., 47 (3), 559-570. [ Links ]
Wang, J., & Anthony, E. J., (2003). A study of thermal - cracking behavior of asphaltenes. Chem. Eng. Scien., 58: 157-162. [ Links ]
Zander, M. (1987). Recent advances in pitch characterization. FUEL, 1987, 66: November. [ Links ]
Zhang Jianzhong, Zhan Biao, & Zhao Suoqui. (1998). Simplified prediction of critical properties of nonpolar compounds, petroleum, and coal liquid fractions. Ind. Eng. Chem. Res., 37: 2059-2060. [ Links ]














