Introduction
Drugs used in medical practice are effective and safe in specific indications. This is achieved through a long and rigorous study process that provides information about the specific population that can be benefited, as well as the use and appropriate dose. However, off-label use of drugs is based on the ability or freedom of physicians who prescribe them beyond approved indications when a therapeutic alternative is required.
Recently, the consumption of psychoactive drugs has increased in Colombia, which is one of the drug groups with the highest proportion of non-approved use, particularly antidepressants, antipsychotics, anxiolytics and sedatives 2-7.
Use beyond approved indications is an issue, and there is no unanimity in this regard among regulatory agencies. In fact, many of them do not even have a clear position. The US Food and Drug Administration (FDA), for example, defines off- label as the "use for indication, dosage form, dose regimen, population or other use parameter not mentioned in the approved labeling" 8.
Formulations different from the approved indication, according to a systematic review by Carton et al.9 based on studies conducted around the world, show that they represent 40-75% of the total prescriptions of psychotropic drugs, mainly for mood, anxiety, insomnia and agitation disorders. Quetiapine is revealed as the main drug used off-label, especially for anxiety and insomnia. The Colombian Food and Drug Surveillance Institute (Invima in Spanish) established through Act 38 of December 13, 2006 10 that "off-label" is a use different from that officially authorized by Invima"; therefore, it refers to unauthorized use and is "only acceptable if supported by clinical studies properly conducted."
In Colombia, little information is known on the unauthorized use of medications, and there is no clear regulation on this issue. For this reason, the following prescription-indication study is presented 11 with the purpose of describing the use of psychoactive drugs beyond the guidelines approved by the regulatory agencies in a health promoting entity (EPS) of Bogota D.C. during the first half of 2010. It is also intended to suggest tools for the regulation and control of medications based on evidence of risk of adverse reactions, lack of effectiveness and inappropriate use of resources.
Materials and methods
Cross-sectional study in which prescription-indication information was collected retrospectively. The sample was obtained from the databases of drugs dispensed by Audifarma S.A. in an EPS of Bogota D.C., as well as from the medical records of the patients included.
From a universe of16 912 patients prescribed with a psychotropic drug for at least one month between January and June 2010, a random sample of 385 patients was classified based on the drugs to be evaluated (expected prevalence: 50%, alpha level: 5%, power: 80%) using the statistical package SPSS v22. Additionally, subjects with lower consumption requirements of diazepam, modafinil and Zolpidem who were not included at first considering the classification criteria, were included later in the study, and, therefore, considered as mandatory, which increased the final size of the sample to 420 individuals.
A list of patients was obtained based on the records of Audifarma S.A. These patients were given one or more of the following psychotropic drugs: valproic acid, alprazolam, amitriptyline, bromazepam, carbamazepine, chlorpromazine, clozapine, diazepam, divalproate, fluoxetine, gabapentin, haloperidol, lamotrigine, lithium, methylphenidate, midazolam, modafinil, olanzapine, oxcarbazepine, pregabalin, risperidone, sertraline, trazodone, venlafaxine and zolpidem. During the same period, a chemist reviewed the medical records to know the medical reason for the prescription. When the drugs with the highest amount of prescriptions were determined, a bibliographical search of the clinical and academic support was made for the different unapproved indications that were found 12.
For the study the following variables were taken into account, and were recorded in a Microsoft Excel database:
Sociodemographic: age (infants <14 years, adolescents 14-18 years, young adults 19-45 years, and adults >45 years) and sex. Clinical: diagnosis and indication for prescription. Pharmacological: name of prescribed medication, route of administration, posology, pharmaceutical form and specialty of the prescribing physician.
Concordance: correlation between the use indication of the psychoactive drug and the indication approved by Invima and FDA (indication, dose, route of administration, contraindications).
Finally, each prescription was classified as a) off-label: use other than the approved by the regulatory agency; b) approved use: use under approved conditions; c) no report: use is not specified in the medical record, and d) not evaluable: no information that supports the use of the psychoactive drug and impossibility to evaluate whether the prescription is appropriate or not (this option was only applied for mandatory inclusion drugs because of the impossibility of replacing the information).
This study met the specifications of the category "research without risk" according to Resolution 8430 of 1993 of the Ministry of Health of Colombia 13, which establishes the scientific, technical and administrative standards for health research. The principles of beneficence and confidentiality of patients were preserved as established by the Declaration of Helsinki.
Analysis plan
Data were analyzed using the statistical package SPSS Statistics version 22.0 for Windows (IBM, U.S.A). A description of frequencies and proportions was made for categorical variables, and central tendency and dispersion measures for continuous variables. Chi-square tests were used for comparing categorical variables, using a variable dependent on off-label use of a drug. Binary logistic regression models were used based on the prescription not approved by Invima as a dependent variable (because it was of greater local interest), and p<0.05 was determined as the level of statistical significance.
Results
420 clinical histories of patients prescribed with psychoactive drugs in Bogotá D.C were evaluated. The mean age was 44.2±18.8, and distribution was obtained with a female predominance of 67.9%.
Table 1 shows the distribution by sex, age group, specialty of the prescribing physician and method of use, as well as the proportion of off-label use.
Table 1 Socio-demographic variables, prescribing physician and off-label indications of psychoactive drugs in patients affiliated with a health promoting entity. Bogotá D.C. 2010.
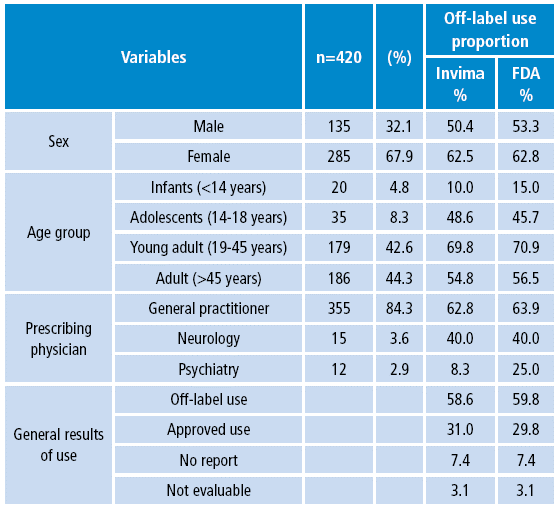
Source: Own elaboration based on data obtained in the study.
It was not possible to describe the use of drugs by route of administration different from that recommended by the health agency, because 31.4% of prescriptions did not report this variable for each psychoactive drug.
Table 2 lists the seven most frequent formulations of psychotropic drugs, and details the proportion of off-label prescriptions and the diagnostic reasons for off-label use.
Table 2 Psychopharmaceuticals most commonly used in off-label indications in patients affiliated with a health promoting entity. Bogotá D.C. 2010.
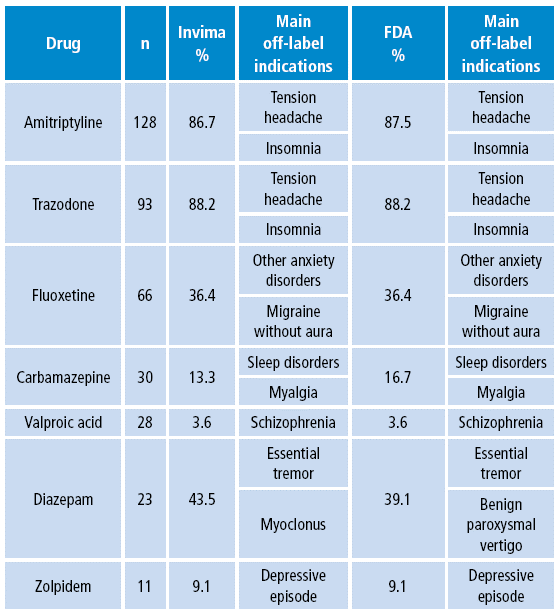
Invima: Food and Drug Surveillance Institute; FDA: Food and Drug Administration.
Source: Own elaboration based on data obtained in the study.
Bivariate analysis
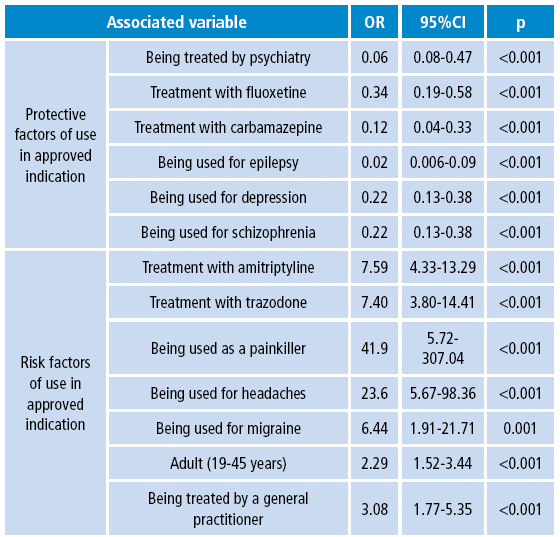
When comparing the dependent variable "off-label prescription not approved by Invima", the variables prescribing a psychoactive drug as a painkiller and being treated for headache with psychoactive drugs were found to be the most likely reasons for off-label indications. However, other variables such as prescription of amitriptyline or trazodone, receiving psychotropic drugs for migraine, being a young adult, and being formulated by general practitioners increased the probability of off-label prescriptions. Interestingly, the total number of patients treated for pain management (p=0.0001) and insomnia (p=0.0001) with psychotropic drugs were prescribed with drugs based on off-label indications.
It was also found that the use of psychoactive drugs for the treatment of epilepsy and being treated by psychiatry were the variables that further reduced the risk of unapproved use, although, prescription with fluoxetine or carbamazepine, depression and schizophrenia, being male and being an infant were associated with a lower probability of using psychoactive drugs with an off-label indication.
Table 3 presents the associated variables in relation to the decrease or increase of the risk of prescribing off-label indications of drugs.
Multivariate analysis
By including the associated variables in a statistically significant manner, a binary logistic regression was performed. It is presented in Table 4, with the variables that were statistically associated with a higher risk of off-label prescriptions according to the approval of Invima.
Table 4 Multivariate analysis of the variables associated with off-label prescription of psychotropic drugs in patients affiliated with a health promoting entity. Bogotá D.C. 2010.

B: Regression coefficient; SE: standard error; DF: degree of freedom; Sig: level of significance. Source: Own elaboration based on data obtained in the study.
Discussion
This study served to identify the most common use of psychoactive drugs used beyond the approval of regulatory agencies and the FDA, as well as the diagnostic reasons, the prescriptive specialty and the variables associated with this practice in an EPS of Bogotá D.C. This contribution is the first approach to information regarding the unapproved use of psychotropic drugs in Colombia.
The proportion of psychoactive drugs found in this study (58.6%), especially antidepressants in non-approved indications, is within the range published by other authors such as Kharadi et al.14 (39.5%, predominantly benzodiazepines), Leslie et al.7 (60.2%) and Chen et al.15 (75.4% predominantly antidepressants).
As presented in the systematic review of Carton et al.9, off-label prescriptions correspond to 40-75% of total prescriptions of psychotropic drugs worldwide according to the reviewed studies, which coincides with the data presented in this study. In this review, the most frequent uses of off-label medications are mood, anxiety and insomnia disorders. However, this research showed that they were almost always used for the management of tension headache and insomnia, being amitriptyline and trazodone the most used drugs, compared with quetiapine as the main drug in this systematic review 9. These differences may be explained by the access to medicines in the Colombian health system, where the prescription of many of these psychotropic drugs is restricted, although amitriptyline and trazodone are over-the-counter.
Off-label drug use may be justified in cases that require therapeutic alternatives, for example, in population groups with few investigations such as infants or pregnant women 1. However, statistical analyzes show that these prescriptions were more frequent in young adults, which may be related to the lack of knowledge by the general practitioner of the indications authorized for the drugs, the population that uses these type of drugs, and those easily related to diagnoses with other approved alternatives 1,2,8.
This scenario leads to reflect on whether the physician, when prescribing, makes an appropriate analysis of the risk-benefit balance based on available clinical evidence and on the Invima guidelines that establish that unauthorized use is acceptable only if it is supported by adequate clinical studies 10.
Amitriptyline and trazodone were frequently prescribed off-label to treat tension headache and insomnia. Regarding amitriptyline, some studies have reported its effectiveness in these two indications 14-17; however, regulatory agencies still do not approve these uses. Something similar occurs with trazodone when indicated to treat sleep disorders. Some reports show good results, but it has not been approved for it yet.
This situation creates a gap between the clinical practice guidelines that include it, and the surveillance and regulation measures that have not been updated. As a consequence, the responsibility of prescribing falls directly on physicians without the support of the drug regulatory agencies 17,18.
Amitriptyline, trazodone, fluoxetine, carbamazepine and valproic acid are less likely to be used without following the guidelines in patients with depression or epilepsy, but may increase the likelihood of unapproved use in different indications, although they may be effective 16,18.
Given the results presented in this paper, questions are raised about the relevance of unapproved prescriptions, since the panorama of off-label drug use does not offer conclusive answers. Perhaps, the best approach to a more rational use of drugs is based on the availability of resources -drugs and other technologies-, the robustness and availability of scientific evidence, the expertise of the physician, and the competence of the surveillance authorities 1.
Therefore, the efforts of all health professionals, regulatory agencies and the pharmaceutical industry should be focused on working together to ensure patient safety, the collection of scientific evidence and an informed prescription. This translates into a more rational use of drugs 16,17.
One of the limitations of this work is its results, which were obtained from a single health promoting institution and can be extrapolated to populations with similar characteristics. In addition, the information was collected from the clinical records and the drug dispensing database, but not through interviews with physicians who are the actual decision makers and are responsible of selecting the most appropriate therapy for their patients. However, the rigor of data collection allows us approach the reality of the use of psychoactive drugs in indications not authorized by regulatory agencies.
In conclusion, psychoactive drugs, especially antidepressants and antiepileptics, are used in this cohort of patients in a high proportion in indications not approved by regulatory agencies such as Invima and FDA, and were mostly prescribed by general practitioners for treating headaches and insomnia. It is important to strengthen information and continuing education programs, as well as relevant updates of health records of some psychotropic drugs with precise indications that already have sufficient evidence. Similarly, surveillance and control strategies should be developed for these drugs or included in pharmacovigilance programs by regulatory agencies when their uses may compromise patient safety in order to minimize the risks related to incomplete or inadequate information that may lead to increased off-label use of medications 18.