Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Colombiana de Entomología
versión impresa ISSN 0120-0488versión On-line ISSN 2665-4385
Rev. Colomb. Entomol. v.32 n.1 Bogotá ene./jun. 2006
Foraging activity of the solitary andean bee, Anthophora walteri (Hymenoptera: Apidae, Anthophorini)
Actividad de forrajeo de la abeja andina solitaria, Anthophora walteri (Hymenoptera: Apidae,Anthophorini)
VÍCTOR H. GONZALEZ1, BERNARDO MANTILLA2, ELIANA PALACIOS2
1 Department of Ecology and Evolutionary Biology, Snow Hall, 1460 Jayhawk Boulevard, University of Kansas, Lawrence, Kansas 66045-7523; e-mail: vhgonza@ku.edu
2 Departamento de Biología. Universidad Javeriana. Santa Fé de Bogotá, Colombia; e-mail: bernarmantilla@hotmail.com; eliana_p_p@hotmail.com
Abstract. This note reports observations on the pollen collecting behavior and foraging activity of the solitary bee, Anthophora walteri Gonzalez on Salvia bogotensis in the Eastern Andes of Colombia. Bees foraged from 7:00–17:00 h, or when the temperature exceeded 15°C. Peak visits occurred between 8:00–9:00, when the temperature was about 18°C and the humidity was 60%. On average, bees spent 3 seconds at each flower and collected pollen throughout the day, although pollen-collecting trips were twice as frequent in the morning as in the afternoon. The daily number and duration of foraging trips per bee ranged from 1–13 trips (x = 6.8 ± 4.3) and 4–88 min (x = 21.7 ± 23.8). Some possible morphological and behavioral adaptations for pollen collection on flowers of Salvia, as well as thermal constraints on the foraging activity of A. walteri in the Andes are also discussed.
Key words: Anthophora walteri. Foraging behavior. Salvia. Andes. Colombia.
Resumen. En esta nota se registran observaciones del comportamiento de recolección de polen y actividad de forrajeo de la abeja solitaria, Anthophora walteri Gonzalez sobre Salvia bogotensis en la cordillera Oriental de Colombia. Las abejas forrajearon desde las 7:00–17:00 h o cuando la temperatura superó los 15°C. El pico de visita ocurrió entre 8:00–9:00, cuando la temperatura fue aproximadamente 18°C y la humedad 60 %. En promedio, las abejas gastaron 3 segundos por flor y colectaron polen todo el día; sin embargo, los vuelos de colecta de polen fueron dos veces más frecuentes en la mañana que en la tarde. El número diario y duración de los vuelos de forrajeo por abeja variaron de 1–13 viajes ( x = 6.8 ± 4.3) y 4–88 min ( x = 21.7 ± 23.8). También se discuten algunas adaptaciones morfológicas y comportamentales para la recolección del polen de flores de Salvia, como también el efecto de las condiciones climáticas de los Andes sobre la actividad de forrajeo de A. walteri
Palabras clave: Anthophora walteri. Comportamiento de forrajeo. Salvia. Andes. Colombia.
Introduction
Anthophora (Mystacanthophora) walteri Gonzalez 2004 (Hymenoptera: Apidae, Anthophorini) is a solitary bee species, about the size of a honeybee worker (12– 15 mm long), endemic to isolated xeric highlands (2000–3000 m) in the Eastern Andes of Colombia (Gonzalez & Chavez 2004; Gonzalez & Engel 2004; Gonzalez et al. 2005). Nests are usually found in aggregations of 5–65 nests per square meter in flat ground or small banks in very hard soils in open, barren areas. As in other species of the subgenus Mys-tacanthophora, and certain bee genera in the families Andrenidae, Colletidae and Megachilidae, females of A. walteri have a face with a flat supraclypeal area and a clypeus covered with apically hooked hairs. Such modified hairs are apparently related to pollen collection from Salvia (Lamiaceae) (Michener 2000; Gonzalez & Chavez 2004). Palynological analysis of A. walteri brood provisions in Mondoñedo (Departamento of Cun-dinamarca, Colombia) showed that nearly all pollen within each nest cell was from Salvia bogotensis Bentham, 1824 (Gon-zalez & Chavez 2004). Because little is known about the foraging ecology of A. walteri or any neotropical Anthophora, herein we report observations on its for-aging and pollen-collecting behavior. Possible morphological and behavioral adaptations to pollen collection on flow-ers of Salvia, as well as the effect of cli-matic conditions on its foraging activity, are also discussed.
Materials and Methods
This study was conducted on December 18–20, 2004, in Mondoñedo (4° 39 52.9 N; 74° 17 2 W), about 10 km W of Bogotá city (Cundinamarca, Colombia), at 2720 m. Mondoñedo is a semiarid area that is highly disturbed by cattle ranching, gravel extraction and waste dumping. The rainy season is bimodal with a maximum in April–June and the other in September–October, never reach-ing above 100 mm of precipitation monthly. The median temperature is ap-proximately 13 °C. The most predomi-nant plant is Salvia bogotensis, a small shrub (< 1 m tall) that provides sparse but spatially aggregated shade in the area (Gonzalez & Chavez 2004).
The daily foraging activity of A. walteri was studied at a nest aggregation con-sisting of 21 nests per square meter, and on three patches (8–9 m2 each) of S. bogotensis. Early in the morning, before bees started to forage, nests entrances were marked with small pieces of paper (~ 2x2 cm), which were attached to the ground with an insect pin. The departure and arrival times were recorded for each bee, as well as the presence or absence of pollen on the scopae. Pollen carrying females were easy to distinguish by the yellow pollen loads on their hind leg scopae. Unlike arrivals, bees left the nest very quickly and did not exhibit an ori-entation flight at the nest entrance; thus, it was difficult to observe the exact de-parture time in some cases. We therefore only used data on arrival times to build figure 3. Observations were made about 1.0–1.5 m away from the nest aggregation.
At the flowers, the numbers of A. walteri individuals were recorded every 30 minutes from 6:00 to 18:00 during a 10 min scan sampling (n = 2 h total) on Dec. 20, 2004. When possible, focal observations were taken from marked females (n = 34) that were uniquely painted on the mesosoma with quick-drying Decocolor ® paint markers 24 h before observations. Bees were captured on the selected patches with an insect net and placed into plastic vials and immobilized on ice for about 3–4 min. Once marked, bees were released on the same patch where they had been captured. Ambient temperature and relative humidity taken in shade were recorded using a digital hygrother-mometer (Extech®).
We used a linear regression analysis to test the effect of ambient temperature and relative humidity on the frequency of bee visits. Mean values are given with standard deviations.
Results
Pollen collecting behavior. Bees landed on S. bogotensis flowers with the tongue extended and held onto the petals with the anterior and middle legs. Then, they introduced the tongue into the corolla while quickly moving the head back and forth. Bees spent on average 3 seconds per flower (± 1.0, n = 19 bees), although visits seemed to be shorter when another bee had previously visited the same flower. After visiting several flowers, a female would fly to a tiny branch of S. bogotensis or a dead branch of a nearby plant and gripped it firmly with her mandibles. Then, she would raise her body and used the forelegs to remove pollen from her face while actively moving her body laterally and passing the pollen to the middle legs and finally, to the hind tibial scopae. Bees spent between 5–40 seconds ( = 17 s ± 1.0, n = 5) loading pollen onto scopae. Afterwards, bees im-mediately returned to foraging or re-mained motionless for a few minutes (1–4 min) (n = 3). The exact number of flowers that a female required before transferring pollen to her scopae was difficult to es-tablish because bees did not stay long enough in the patch or because they al-ready had pollen on their scopae at the beginning of observation. However, in one instance, a female visited 130 flow-ers in 419 s (~ 7 min) before we observed her transferring pollen onto her scopae. Unlike bees without pollen, pollen-car-rying bees visited inflorescences in a more organized fashion, starting from the bottom and visiting almost every flower while moving up to the top.
= 17 s ± 1.0, n = 5) loading pollen onto scopae. Afterwards, bees im-mediately returned to foraging or re-mained motionless for a few minutes (1–4 min) (n = 3). The exact number of flowers that a female required before transferring pollen to her scopae was difficult to es-tablish because bees did not stay long enough in the patch or because they al-ready had pollen on their scopae at the beginning of observation. However, in one instance, a female visited 130 flow-ers in 419 s (~ 7 min) before we observed her transferring pollen onto her scopae. Unlike bees without pollen, pollen-car-rying bees visited inflorescences in a more organized fashion, starting from the bottom and visiting almost every flower while moving up to the top.
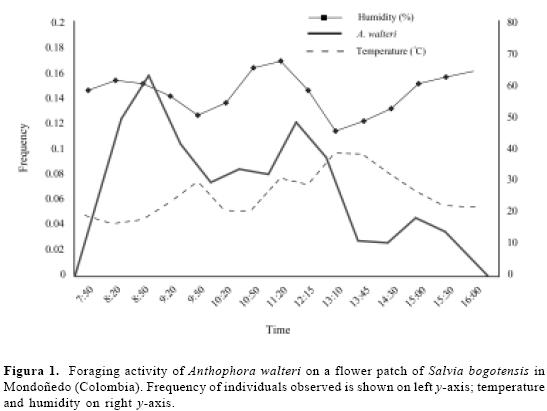
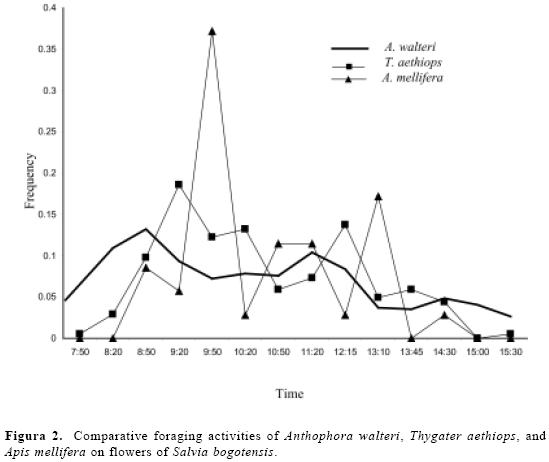
Foraging activity. About 11 % of all A. walteri individuals (total n = 597) ob-served on S. bogotensis flowers were males. Females visited flowers for pollen and nectar whereas males visited just for nectar and to chase females for mating. During the study period, atmospheric temperature ranged 15.3–25.7 °C (x= 20.8, ± 0.1, n = 15) and humidity 46–68 % (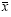 = 58.3 ± 0.1, n = 15). Bees started to forage as early as 7:00 or, on cooler days, when temperature reached above 15 °C. No bees were seen on flowers or entering or leaving the nest after 17:00. Males and females of the solitary bee, Thygater aethiops (Smith, 1854) (n = 204 indi-viduals) (Apidae, Eucerini), and honey bee workers Apis mellifera Linneaus, 1758 (n = 135) (Apidae, Apini) were also frequently seen on S. bogotensis flow-ers. Queens and workers of the bumble bee, Bombus atratus Franklin, 1913 (Apidae, Bombini) also sporadically (n = 17) visited flowers of S. bogotensis throughout the day. All of these bees vis-ited S. bogotensis flowers for nectar. The highest visit peak of A. walteri on S. bogotensis occurred between 8:00–9:00. A second peak occurred late in the morn-ing (11:00–12:30); in both cases, tem-perature was low and humidity high, contrasting with the activity peak of T. aethiops and A. mellifera, which occurred in periods when temperature was high and humidity low (Figs. 1, 2). There were not, however, a significant effect (P > 0.05) of the ambient temperature and relative humidity on the visit frequencies of A. walteri, T. aethiops and A. mellifera on flowers of S. bogotensis. During strong winds or low temperatures (< 15 °C) some bees remained motionless on flowers or among leaves until wind ceased or tem-perature increased.
= 58.3 ± 0.1, n = 15). Bees started to forage as early as 7:00 or, on cooler days, when temperature reached above 15 °C. No bees were seen on flowers or entering or leaving the nest after 17:00. Males and females of the solitary bee, Thygater aethiops (Smith, 1854) (n = 204 indi-viduals) (Apidae, Eucerini), and honey bee workers Apis mellifera Linneaus, 1758 (n = 135) (Apidae, Apini) were also frequently seen on S. bogotensis flow-ers. Queens and workers of the bumble bee, Bombus atratus Franklin, 1913 (Apidae, Bombini) also sporadically (n = 17) visited flowers of S. bogotensis throughout the day. All of these bees vis-ited S. bogotensis flowers for nectar. The highest visit peak of A. walteri on S. bogotensis occurred between 8:00–9:00. A second peak occurred late in the morn-ing (11:00–12:30); in both cases, tem-perature was low and humidity high, contrasting with the activity peak of T. aethiops and A. mellifera, which occurred in periods when temperature was high and humidity low (Figs. 1, 2). There were not, however, a significant effect (P > 0.05) of the ambient temperature and relative humidity on the visit frequencies of A. walteri, T. aethiops and A. mellifera on flowers of S. bogotensis. During strong winds or low temperatures (< 15 °C) some bees remained motionless on flowers or among leaves until wind ceased or tem-perature increased.
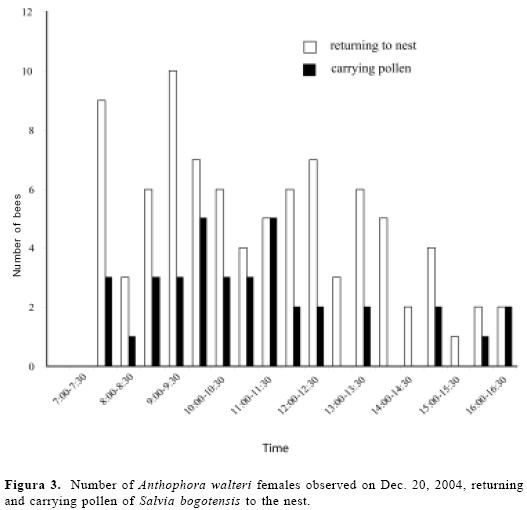
The daily number and duration of forag-ing trips per bee female ranged 1–13 trips per day (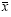 = 6.8 ± 4.3, n = 10 bees) and 4–88 min per trip (x= 21.7 min ± 23.8, n = 24 trips). Bees collected pollen throughout the day, although the percent-ages of pollen-collecting trips were twice as frequent in the morning as in the after-noon (Fig. 3). Pollen-collecting trips were observed in 42 % of the total observed foraging trips (n = 125). Bees may have carried nectar or water in their crops dur-ing the remaining trips but we did not test this.
= 6.8 ± 4.3, n = 10 bees) and 4–88 min per trip (x= 21.7 min ± 23.8, n = 24 trips). Bees collected pollen throughout the day, although the percent-ages of pollen-collecting trips were twice as frequent in the morning as in the after-noon (Fig. 3). Pollen-collecting trips were observed in 42 % of the total observed foraging trips (n = 125). Bees may have carried nectar or water in their crops dur-ing the remaining trips but we did not test this.
Discussion
All bee species that visited S. bogotensis, including A. walteri, seemed to be attracted to flowers primarily for nectar as indicated by the extended tongue on approach to the flower. Although we do not have data, it is likely that pollen is accidentally transferred to the face in all species during a visit. However, the modified hairs on the faces of A. walteri female may increase the amount of pollen trans-ferred. Most bees, especially hairy bees like B. atratus and T. aethiops, comb off the pollen from the face using the fore-legs during grooming (Thorp 1979; 2000; Roubik 1989; Michener 2000) but the additional repertory of behaviors (i.e., moving the abdomen while holding onto a stem with the mandibles) were only observed in A. walteri. In addition, some of the marked females (n = 8 out of 34) returned several times during the day (1– 4) to forage on the same patch where they had been captured. Anthophora walteri females also seemed to skip flowers that had previously been visited by other bees, suggesting they are capable of rec-ognizing them. All these observations indicate morphological (modified hairs on face) and behavioral adaptations for collecting pollen from Salvia.
The genus Salvia comprises nearly 1000 species worldwide, and they are well known by their modified lever-like sta-mens, which play a key role in pollen transfer (Claßen-Bockhoff et al. 2003). A bee or bird (the main pollinators of Salvia) searching for nectar pushes one of the arms of the modified stamens that blocks the access to nectar, causing pol-len to be loaded onto its head, bill or back. As a result, the pollen may be trans-ferred to the stigma of another flower during a subsequent visit. This type of mechanism is often referred as nototribic pollination (Claßen-Bockhoff et al. 2004). Is A. walteri the only pollinator of S. bogotensis? We do not have data to answer this and related questions regard-ing pollen-transfer mechanisms involved in S. bogotensis. However, given that pollinator availability is very low for plants in high tropical altitudes in com-parison with lower elevations due to climatic conditions (Primack 1985), spe-cialization on a particular type of polli-nator does not seem like a best strategy. Instead, even small contributions from a wide range of pollinators may be advan-tageous to high Andean plants such as S. bogotensis (Fagua & Gonzalez, in press). Undoubtedly, the pollination ecology of S. bogotensis needs to be studied in or-der to address such questions. Similarly, we do not know if A. walteri switches plant host when S. bogotensis is tempo-rally unavailable as has been observed in others pollen specialist bees (Wcislo & Cane 1996).
Tropical high altitude ecosystems such as in Mondoñedo represent climatically hostile environments. Native species must tolerate drastic diurnal temperature changes, freezing temperatures during the night and avoid desiccation during the day (Sarmiento 1986; Lüttge 1997). Low temperatures and inclement weather strongly influence flight activity of heterothermic animals such as bees. Therefore, foraging is primarily restricted to warmer daily periods and to those ani-mals that can efficiently thermoregulate their body temperature (Bishop & Armbruster 1999). We did not find a sig-nificant effect of the ambient tempera-ture and relative humidity on the foraging activity of A. walteri though we only have data from a single day. Nonetheless, A. walteri did not fly at temperatures below 15 °C, during light rain or even before sunrise as frequently reported for some temperate Anthophora species [e.g., A. plumipes (Pallas, 1772), and A. bomboi-des stanfordiana Cockerell, 1904] (Brook 1983; Batra 1994; Stone 1994). This agrees with the idea that unlike ar-thropods from temperate areas, tropical alpine arthropods apparently do not have time for physiological preparation before the onset of low temperatures (Sømme 1989; Sømme et al. 1996). Furthermore, diurnal changes in quality and quantity of available floral nectar and pollen in S. bogotensis may also influence A. walteri activity as noted for other oligolectic Anthophora species (Stone et al. 1999).
In addition to climatic conditions, the number and duration of foraging trips per day of bees may also depend on individual status in social species, nest ac-tivities (cell construction, provisioning), type of material collected (mud, pollen, nectar), resource distance, etc. The daily number and length of trips observed in A. walteri are within the range of trips reported for other social and solitary spe-cies (e.g., Michener 1974). Spending time away from the nest while foraging for food could increase the risk of brood parasiti-zation, especially in solitary species. Anthophora walteri females spent as much as 7 min foraging on 130 flowers before loading pollen in the scopae; that is, they could visit more than 500 flow-ers to complete a full pollen load on each trip, and spend on average, at least, 3.5 h per day (~200 min) away from the nest. Unlike habitats containing Anthophora species in the Northern Hemisphere, no nest parasites (including parasitic bee species) are known to occur in Mon-doñedo (Gonzalez & Chavez 2004). Al-though we did not mark the bees from the nest aggregation under study, it was clear that sometimes a non-resident fe-male entered a nest when it was already occupied. Buzzing and aggressive en-counters usually occurred, and presum-ably those non-resident bees, then left the nest. Such intra-specific competition has also been reported in other Anthophora species (Batra 1994).
Acknowledgments
This paper is dedicated to Mr. Mauricio Palacios and Mrs. Rita Morillo, beloved parents of E. Palacios, for their encour-agement, patient and unconditional sup-port during our fieldwork in Mondoñedo. We thank Petra Wester, Daniel Bennett, Carlos Sarmiento, Marisol Amaya, and three anonymous reviewers for comments on the manuscript. Finally, we gratefully acknowledge the financial support of Idea Wild (to VHG). This is a contribu-tion of the Division of Entomology, Natural History Museum and Biodiversity Research Center, University of Kansas.
References
BATRA, S. W. 1994. Anthophora pilipes villosula Sm. (Hymenoptera: Antho-phoridae), a manageable Japanese bee that visits blueberries and apples during cool, rainy, spring weather. Proceedings of the Entomological Society of Washington 96(1): 98–119. [ Links ]
BISHOP, J. A.; S. ARMBRUSTER. 1999. Thermoregulatory abilities of Alaskan bees: effects of size, phylogeny and ecol-ogy. Functional Ecology 13: 711–724. [ Links ]
BROOK, R. W. 1983. Systematics and bio-nomics of Anthophora: The Bomboides group and species group of the New World (Hymenoptera: Apoidea, Anthophoridae). University of California, Publications in Entomology 98: 1–86. [ Links ]
CLAßEN-BOCKHOFF, R.; WESTER, P.; E. TWERASER. 2003. The staminal lever mechanism in Salvia L. (Lamiaceae) – a review. Plant Biology 5: 33–41. [ Links ]
CLAßEN-BOCKHOFF, R.; SPECK, T.; TWERASER, E.; WESTER, P.; THIMM, S., & M. REITH. 2004. The staminal le-ver mechanism in Salvia L. (Lamiaceae): a key innovation for adaptive radiation? Or-ganisms, Diversity and Evolution 4: 189-205. [ Links ]
FAGUA, C.; V. H. GONZALEZ. In press. Growth rates, reproductive phenology and pollination ecology of Espeletia grandiflora (Asteraceae). Biotropica. [ Links ]
GONZALEZ, V. H.; F. CHAVEZ. 2004. Nest-ing biology of a new high Andean bee, Anthophora walteri Gonzalez (Hymenoptera:Apidae: Anthophorini). Journal of the Kan-sas Entomological Society 77(4): 36–44. [ Links ]
GONZALEZ, V. H.; M. S. ENGEL. 2004. The Tropical Andean bee fauna (Insecta: Hy-menoptera: Apoidea), with examples from Colombia. Entomologische Abhandlungen 62(1): 65–75. [ Links ]
GONZALEZ, V. H.; OSPINA, M.; D. BENNETT. 2005. Abejas altoandinas de Colombia: Guía de campo. Instituto de Investigación de Recursos Biológicos Al-exander von Humboldt, Bogotá, D.C., Colombia. 80 pp. [ Links ]
LÜTTGE, U. 1997. Physiological Ecology of Tropical Plants. Springer-Verlag Berling Heidelberg, Germany. 384 pp [ Links ]
MICHENER, C. D. 1974. The social behavior of bees. Harvard University Press; Cambridge, xii+ 404 pp. [ Links ]
MICHENER, C. D. 2000. The Bees of the World. Johns Hopkins University Press; Baltimore, MD; xiv+[1]+913 pp. [ Links ]
PRIMACK, R. 1985. Longevity of individual flowers. Annual Review of Ecology and Systematics 16: 15–37. [ Links ]
ROUBIK, D. W. 1989. Ecology and natural history of tropical bees. Cambridge Uni-versity Press, Cambridge, 514 pp. [ Links ]
SARMIENTO, G. 1986. Ecological features of climate in high tropical mountains. Pp. 11–45 in F. Vuilleumier., and M. Monasterio, editors. High Altitude Tropical Bio-geography. Oxford University Press, Oxford, [ Links ]
SØMME, L. 1989. Adaptation of terrestrial arthropods to alpine environments. Bio-logical Reviews 64: 367–407. [ Links ]
SØMME, L.; R. L. DAVIDSON.; G. ONORE. 1996. Adaptations of insects at high altitudes of Chimborazo, Ecuador. European Journal of Entomology 93: 313–318. [ Links ]
STONE, G. N. 1994. Patterns of evolution of warm-up rates and body temperatures in flight in solitary bees of the genus Anthophora. Functional Ecology 8: 324– 335. [ Links ]
STONE, G. N.; F. GILBERT.; P. WILLMER.; S. POTTS.; F. SEMIDA; S. ZALAT. 1999. Windows of opportunity and the temporal structuring of foraging activity in a desert solitary bee. Ecological Entomology 24: 208–221. [ Links ]
THORP, R. W. 1979. Structural, behavioral, and physiological adaptations of bees (Apoidea) for collection pollen. Annals of the Missouri Botanical Garden 66(4): 788–812. [ Links ]
THORP, R. W. 2000. The collection of pollen by bees. Plant systematics and evolution 222: 211–223. [ Links ]
WCISLO, T.; J. CANE. 1996. Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stored foods by natural enemies. Annual Review of Entomology 41: 257-286. [ Links ]
Recibido: 19-jul-05 Aceptado: 12-dic-05