Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Colombiana de Entomología
versión impresa ISSN 0120-0488versión On-line ISSN 2665-4385
Rev. Colomb. Entomol. v.37 n.2 Bogotá jul./dic. 2011
Scientific note
Emergence patterns of Orgyia ericae (Lepidoptera: Lymantriidae) parasitoids
Patrones de emergencia de los parasitoides de Orgyia ericae (Lepidoptera: Lymantriidae)
CUI YA-QIN1, SHENG MAO-LING2, LUO YOU-QING3, and ZONG SHI-XIANG4 1 Dr. Cui Ya-Qin, Beijing Forestry University, Beijing Forestry University, Beijing, 100083, P. R. China. sxtycyq@foxmail.com. 2 Prof. Dr. Sheng Mao-Ling, General Station of Forest Pest Management, State Forestry Administration, Shenyang 110034, P.R. China. shengmaoling@163.com. 3 Prof. Dr. Luo You-Qing, Beijing Forestry University, Beijing Forestry University, Beijing, 100083, P. R. China. youqingluo@126.com. 4 Vice-Prof. Dr. Zong Shi-Xiang, Beijing Forestry University, Beijing Forestry University, Beijing, 100083, P. R. China. zongsx@126.com. Author for correspondence. Received: 19-may-2010 - Accepted: 31-mar-2011
Abstract: To provide guidance on the utilization and conservation of the forest defoliator Orgyia ericae parasitoids, we survey its parasitoid species. We identifed the developmental stages associated with the different parasitoids, and the emergence pattern of adult parasitoids. Observations of hatching were conducted hourly from 7am to 12pm until all adults had emerged. Pupation time, emergence patterns, and the abundance of Exorista larvarum parasitoids were recorded in detail. The abundance and daily emergence patterns of Bracon (Habrobracon) sp., and Tetrastichus sp. were analyzed. Bracon (Habrobracon) sp. and E. larvarum both had only one emergence peak, whereas Tetrastichus sp. had two. The daily emergence of Bracon (Habrobracon) sp., Tetrastichus sp. and E. larvarum peaked at 3 to 5pm, 3 to 5pm and 7 to 8am, respectively. The E. larvarum pupation distribution was characterized by two peaks.
Key words: Parasitoids. Bracon (Habrobracon) sp. Tetrastichus sp. Exorista larvarum. Grey-spotted tussock moth.
Resumen: Para proveer guías acerca de la utilización y conservación de los parasitoides del defoliador forestal Orgyia ericae se inventariaron sus especies de parasitoides. Se identificaron los estados de desarrollo del lepidóptero con las distintas especies de parasitoides así como sus patrones de emergencia. Se adelantaron observaciones de emergencia desde las 7am hasta 12pm hasta que todos los adultos emergieron. Tiempo de pupación, patrones de emergencia y abundancia del parasitoide Exorista larvarum se registró en detalle. Se analizaron la abundancia y patrones de emergencia de Bracon (Habrobracon) sp. y Tetrastichus sp. Bracon (Habrobracon) sp. y E. larvarum exhibieron un solo pico de emergencia mientras que Tetrastichus sp. muestra dos picos. La emergencia diaria de Bracon (Habrobracon) sp., Tetrastichus sp. y E. larvarum fue mayor entre las 3 a 5pm, 3 a 5pm y 7 a 8am, respectivamente. La pupación de E. larvarum mostró dos picos.
Palabras clave: Parasitoides. Bracon (Habrobracon) sp. Tetrastichus sp. Exorista larvarum. Polilla de penacho de manchas grises.
Introduction Orgyia ericae (Germar, 1824) is a defoliator moth of the Lymantriidae family (Lepidoptera) (Xu 1980; Wu et al. 1982). It is distributed mainly in Heilongjiang, Jilin, Liaoning, Shaanxi, Gansu, Qinghai, and Shandong provinces, in the autonomous regions of Inner Mongolia and Ningxia in China, and in the former Soviet Union and Europe (Zhao 1978; Xiao 1992; Dapkus 2004, 2010). O. ericae is an important pest of trees. Given the economic and ecological importance of the species it attacks, O. ericae has a major impact in the regions where it is found. In recent years, it has been frequently reported to attack Hedysarum fruticosum Pall. var. mongolicum (Turcz. ex B. Fedtsch., 1902) (Fabaceae), Caragana, Hedysarum scoparium (Fisch. et C. A. Mey., 1841) (Fabaceae), Calligonum mongolicum (Turcz., 1832) (Polygonaceae), Ammopiptanthus mongolicus (Maxim. ex Kom.) (S. H. Cheng, 1959) (Fabaceae) and other desert plants, occurring in a large area of desert shrubbery of regions such as Inner Mongolia, Ningxia and Qinghai. These species are important for desert shrubbery in northwest China, and play a particularly key role in landscape stability and desertification control. They are resistant to drought, sand burial, wind erosion, sand, and barren soil, and grow rapidly. These plants can be used as fodder for livestock (except A. mongolicus), and as ingredients for Chinese medicine, also edible oils can be extracted from seeds of these plants (Liu et al. 1985; Liu et al.1987). Studies of the biological characteristics and life history of O. ericae have shown that O. ericae has two generations per year (Xu 1980; Xiao 1992; Wang & Liu 2002; Wang et al. 2009). Individuals overwinter as eggs in cocoons, which hatch in the middle of May. The newly hatched larvae undergo six instars of four to fve days each, usually feeding on leaves. The dispersion of larvae depends on wind. Pupation begins in mid June on the branches of plants, and adults emerge after approximately ten days, in early July. The wingspan is 21-28mm for males, while females are wingless and release sex pheromones that attract mates to cocoons, where eggs are laid. Each female adult can produce, on average, approximately 250 eggs. Males have obvious phototaxis. The second generation of eggs begins to hatch in the middle of July, with pupation beginning in mid to late August and adult emergence in early September. The eggs overwinter in cocoons after mating. Although the biological characteristics and parasitoids of O. ericae (Wang & Liu 2002; Yu et al. 2007; Wang et al. 2008; Sun et al. 2008; Li et al. 2009), and the sex pheromone (Chen et al. 2010; Chen et al. 2011), nuclear polyhedrosis virus and polyhedrin gene sequence of of Orgyia ecricae (Zhang et al. 1991; Dai & Zu 1996; Zu & Dai 1997; Yang et al. 2006), have been intensely studied, there are currently no reports describing the emergence patterns of adult O. ericae parasitoids. To provide guidance on the utilization and conservation for parasitoids of O. ericae, we investigated O. ericae parasitoid species and the emergence pattern of adult parasitoids.
Materials and Methods A total of 982 cocoons of O. ericae were collected from H. scoparium in Hangjinqi, Erdos, Inner Mongolia, on July 4, 2008. All cocoons were cultured and observed in two loosely airtight containers placed in a rearing room at 24±1oC, 50±10% relative humidity and a photoperiod of 14:10 (L:D). Observations of O. ericae egg hatching as well as the identification of parasitoid species and measurements of their stage and abundance were conducted hourly from 7am to 12pm from July 7, 2008, until all adults had emerged. In order to represent a proportion of parasitism of all male and female cocoons, all cocoons collected were dissected on August 14, 2008. O. ericae cocoons were also classifed with respect to sex, based on their morphological differences. Female cocoons present one mating hole, and differ in length and color: the female pupa is 15mm long and characterized by a yellow and brown color, whereas the male pupa is ap-proximately 8mm long and it is dark brown. To complete the next stage of their life cycle, the larvae of Exorista larvarum (Linnaeus, 1758) (Tachinidae) (S. M.-L.) must break out of the O. ericae cocoon and pupate. All the pupae of E. larvarum were collected in vitro, and the time of pupation and emergence, as well as the quantity of parasitoids, were recorded. We used LSD multiple comparisons to analyze and identify where differences in the peaks of emergence occurred.
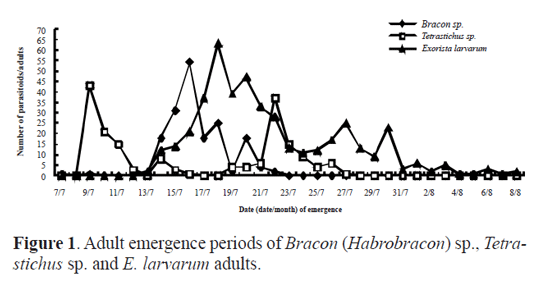
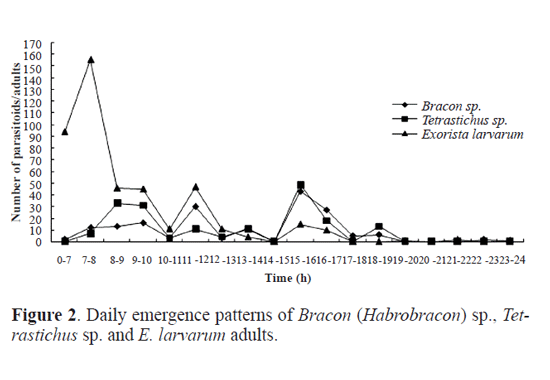
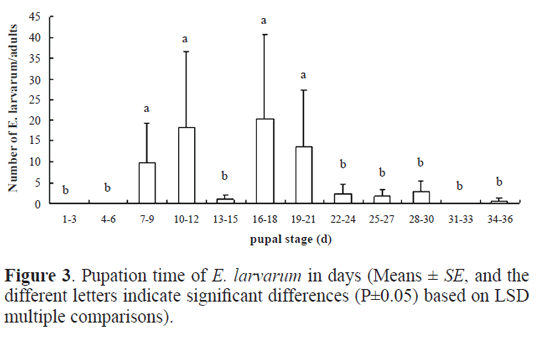
Results Parasitoid species. Out of the 982 cocoons of O. ericae, 485 male cocoons and 384 female cocoons were parasitized, representing a parasitism of 88.18% and 88.89% for male and female cocoons, respectively. Eight parasitoid species were identifed, including the following Hymenoptera, numbers within brackets indicate their abundance: Bracon (Habrobracon) sp. (Braconidae) (174), Itoplectis viduata (Gravenhorst, 1829) (Ichneumonidae) (S. M.-L.) (11), Pimpla disparis (Viereck, 1911) (Ichneumonidae) (S. M.-L.) (3), Eutanyacra picta (Schrank, 1776) (Ichneumonidae) (S. M.-L.) (3), Tetra-stichus sp. (Chalcidoidea) (S. M.-L.) (180), Aprostocetus sp. (Chalcidoidea) (S. M.-L.) (1), Brachymeria lasus (Walker, 1842) (Chalcidoidea) (S. M.-L.) (2), and the Diptera Exorista larvarum (Linnaeus, 1758) (Tachinidae) (445). Adult emergence period of parasitoids. Bracon (Habrobracon) sp. and Tetrastichus sp. were the most frequent Hymenoptera parasitoid species in the sample. By contrast, only 11 adults of I. viduata emerged, and the remaining species of parasitic wasp were represented by three or less individuals. There were 445 adults of E. larvarum in our sample. For Bracon (Habrobracon) sp. it took 11 days for adults to begin to emerge and emergence occurred from the 7th to the 22nd July (Fig.1). Peak emergence corresponded to 54 adults (31% of the total number of Bracon sp. individuals that emerged). Eclosion took 16 days for Tetrastichus sp. and occurred from the 9th to the 27th July (Fig.1). Emergence in this species was characterized by a bimodal pattern of emergence, with the frst peak of emergence being slightly higher than the second. Adults of E. larvarum emerged from mid July to early August (Fig.1). There were three small peaks of emergence concentrated in mid and late July, followed by a low rate of emergence until the 10th August. Daily emergence rhythm of parasitoids. The emergence of Bracon (Habrobracon) sp. peaked between 11 and 12am and between 3 and 5pm, with most emergences occurring between 3 and 5pm (Fig. 2). Tetrastichus sp. emerged most frequently from 7am to 8pm, with two emergence peaks at 8 to 10am and 3 to 5pm, with the second peak being slightly higher (Fig. 2). The daily emergence of E. larvarum was characterized by a high peak of emergence from 7am to 8pm and several low peaks of emergence throughout the day (Fig. 2). Pupation range of Exorista larvarum. E. larvarum pupation lasted from 7 to 34 days and two emergence peaks were observed, accounting for respectively 40% (frst peak) and 48% (second peak) of all emergences (Fig. 3). Our results show that the parasitoid species Bracon (Habrobracon) sp., Tetrastichus sp. and E. larvarum emerged at different times in July, with distinctive differences in the timing of their peaks of eclosion. The consecutive peaks of eclosion may be the consequence of long-term co-evolutionary competition processes among these three parasitoid species, which would have resulted in the minimization of interspecies competition and parasitation of the same host at different periods. This conjecture may also explain how these parasitoid populations are maintained in balance. Although this study has confrmed that the emergence of Tetrastichus sp. adults is characterized by two peaks, it is still not clear whether Tetrastichus sp. is a hyperparasitoid insect. Conversely, a large number of E. larvarum adults emerged between midnight and 7am, but there are no data on the number of eclosions per hour, precluding the determination of specific emergence times. In addition, because the number of individuals belonging to I. viduata, P. disparis, E. picta, Aprostocetus sp. and B. lasus was low, we were unable to determine their patterns of eclosion, an issue that should be addressed in future studies. Most adults of O. ericae emerge in early July, mate and lay eggs. Newly hatched larvae of the second generation emerged in mid July, and mature larval pupated in mid and late August. Adults emerged in early September. Based on the life cycle and habit of O. ericae, Tetrastichus sp. might parasitize eggs of O. ericae, Bracon (Habrobracon) sp. and E. larvarum might attack larvae of O. ericae. Similar results were reported by Haiyan Li et al. (2009), whose anatomical observations of the parasitoids of egg cocoons of O. ericae showed that each developmental stage of O. ericae is parasitized by a different parasitoid species. These findings could permit the selection of the most appropriate parasitoids according to the developmental stage of the host, as well as the selection of suitable prevention and control periods based on adult emergence patterns of parasitoids and the biological characteristics of the host. Chemical control and cocoon extirpation are commonly used to prevent and control O. ericae ( Yu et al. 2007; Sun et al. 2008; Wang et al. 2009; Luan et al. 2010). However, chemical control and cocoon extirpation would also adversely affect parasitoid population numbers, reducing the effectiveness of this natural control of O. ericae. Therefore, it might be appropriate to reduce these artificial control measures during adult emergence periods of parasitoids. Simultaneously, through investigations of O. ericae parasitoid species and the emergence patterns of adult parasitoids, it might be possible to choose a suitable time for the release of parasitoid species into the feld to control population numbers of O. ericae.
Acknowledgments
This research was supported by the Program of the Key Science and Research projects of Chinas State Forestry Administration (Grant No.2006-46), the Fundamental Research Funds for the Central Universities (BLYX200919) and National Natural Science Foundation of China (30730075). We gratefully acknowledge Professor Wang Yi-Ping from Zhejiang Forestry University for helping us to identify Bracon (Habrobracon) sp. (Braconidae) parasitoids.
Cited literature CHEN GF, LI T, SHENG ML, LUAN SS, XU JF, WANG S. 2011. Study on sex pheromone of Orgyia ecricae Germar. China Forestry Science and Technology 25(1): 73-76. [ Links ] CHEN GF, SHENG ML, LI T, MILLAR JG., ZHANG QH. 2010. Synergistic sex pheromone components of the grey-spotted tussock moth, Orgyia ericae. Entomologia Experimentalis et Applicata 136(3): 227-234. [ Links ] DAI MX, ZU AM. 1996. Studies on the constructural polypeptides and nucleic acid of Orgyia ecricae nuclear polyhedrosis virus. Chinese Journal of Biological Control 12 (3): 117-122. [ Links ] DAPKUS D. 2004. Lepidoptera of a raised bog and adjacent forest in Lithuania. European Journal of Entomology 101(1): 63-67. [ Links ] DAPKUS D. 2010. Check-list of butterfies and moths of the notigalė bog (Northern Lithuania). New and rare for Lithuania insect species 22: 91-100. [ Links ] LI HY, ZONG SX, SHENG ML, SU M. 2009. Investigation on the Parasitoids of Orgyia ericae. Scientia Silvae Sinicae 45 (2): 67-70. [ Links ] LIU YX, YANG XL, YAO YY, ZHANG GL. 1985. Flora in Desertis Reipublicae Populorum Sinarum: Tomus 1. Science Publishing House, Beijing, 312p. [ Links ] LIU YX, YANG XL, YAO YY. 1987. Flora in Desertis Reipublicae Populorum Sinarum: Tomus 2. Science Publishing House, Beijing, 178-239p. [ Links ] LUAN SS, XU JF, YANG JJ, LI T, LI HY, GUO ZH. 2010. Comparison of the control effects of two biotic pesticides on Orgyia ericae. Forest Pest and Disease 29(3): 42-43. [ Links ] SUN YW, HUANG CH, LIU L. 2008. Investigation on the biological characteristics and the control experiments of Orgyia ericae. Modern Forestry Science and Technology (4): 73-74. [ Links ] WANG X, LIU Q. 2002. Studies on Orgyia ericae Germara new pest harming endangered plants-Ammopiptanthus mongolicus Cheng f.. Journal of Inner Mongolia Normal University (Natural Science Edition) 31 (4): 374-378. [ Links ] WANG ZJ, HAO XP, YANG ML. 2008. Orgyia ericae Germar and Its Natural Enemy. Journal of Inner Mongolia Forestry Science and Technology 34 (1): 27-28. [ Links ] WANG YF, XU CF, YAO NC, LI CQ, WANG WS, XU ZX. 2009. Biological Characteristics of Harming Seabuckthorns Orgyia Ericae and its Prevention. The Global Seabuckthorn Research and Development 7(1): 38-40. [ Links ] WU FZ, GAO ZN, GAO YY. 1982. Ningxia agricultural entomography: 2nd. Peoples Publishing House, Yinchuan, Ningxia, 150-151p. XIAO GR. 1992. Forest insects of China: the second edition (Re-vised & enlarged). China Forestry Publishing House, Beijing, 1093-1094p. [ Links ] [ Links ] XU ZJ. 1980. Study on Orgyia ericae Germar. Scientia Silvae Sinicae (1): 70-71. [ Links ] YANG LR, WANG D, DUAN LQ, ZHANG CX. 2006. Polyhedrin gene sequence and phylogenetic analysis of a nucleopolyhedro virus isolated from Orgyia ericae Germar. DNA SEQUENCE 17(3): 215-222. [ Links ] YU J, JIA WJ, YANG HJ, LI FR, WANG SQ. 2007. Biological Characteristics of Orgyia ericae Germar and Its prevention and Control Measures. Shaanxi Forestry Science and Technology (4): 124-125, 132. [ Links ] ZHANG B, JIANG JM, ZHAO J. 1991. A preliminary study on a nuclear polyhedrosis virus of Orgyia ecricae. Chinese Journal of Biological Control (2):93. [ Links ] ZHAO ZL. 1978. Economic insect fauna of China: 12th. Science Publishing House, Beijing, 39-40p. [ Links ] ZU AM, DAI MX. 1997. The bioassay and feld evaluation of the nuclear polyhedrosis virus from Orgyia ecricae. Chinese Journal of Biological Control 13(2):57-60 [ Links ]