Servicios Personalizados
Revista
Articulo
Indicadores
 Citado por SciELO
Citado por SciELO  Accesos
Accesos
Links relacionados
 Citado por Google
Citado por Google  Similares en
SciELO
Similares en
SciELO  Similares en Google
Similares en Google
Compartir
Revista Colombiana de Química
versión impresa ISSN 0120-2804
Resumen
MENDOZA, Johanna; RODRIGUEZ, Oscar y AGREDA, Jesús A. THERMOKINETIC STUDY OF THE ZERO, FIRST AND SECOND ORDER REACTIONS IN A PSEUDO-ADIABATIC CALORIMETER: Numerical approach and experimental data. Rev.Colomb.Quim. [online]. 2012, vol.41, n.1, pp.111-122. ISSN 0120-2804.
The signal produced by a pseudo-adiaba-tic calorimeter is simulated by numerical solution of the differential equations that model the chemical kinetics [1], the ther-mal properties of the calorimetric cell [2], and the response of the thermistor used as a thermometric sensor [3]. 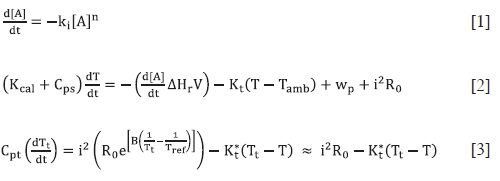 These equations show that the calorimetric signal is related with concentration in a complex way. Therefore, a compa-rison between the signáis of the three basic kinetics reactions (zero, first and second order) was made, as a first step to obtain a standard procedure to follow chemical kinetics using a calorimeter. In order to help understanding this relation-ship, the initial rate method was applied to the simulated data to assess the rela-tionship between the order and the kine-tic constants calculated with those used for the simulations. As it was expected, the initial rate method for the calorime-tric data, do not give a slope directly re-lated with the order of the reaction, as it would be produced, for example, in data from a spectrophotometer. However, a linear relationship was found between what we call the "calorimetric order" and the kinetic order. Finally, the deve-loped procedure was applied to the stu-dy of the H2O2 decomposition catalyzed with Fe3+ in homogeneous phase and with activated carbon in heterogeneous phase, fnding the order and the kinetics constants of the global processes, which were in close agreement with those in the literature.
These equations show that the calorimetric signal is related with concentration in a complex way. Therefore, a compa-rison between the signáis of the three basic kinetics reactions (zero, first and second order) was made, as a first step to obtain a standard procedure to follow chemical kinetics using a calorimeter. In order to help understanding this relation-ship, the initial rate method was applied to the simulated data to assess the rela-tionship between the order and the kine-tic constants calculated with those used for the simulations. As it was expected, the initial rate method for the calorime-tric data, do not give a slope directly re-lated with the order of the reaction, as it would be produced, for example, in data from a spectrophotometer. However, a linear relationship was found between what we call the "calorimetric order" and the kinetic order. Finally, the deve-loped procedure was applied to the stu-dy of the H2O2 decomposition catalyzed with Fe3+ in homogeneous phase and with activated carbon in heterogeneous phase, fnding the order and the kinetics constants of the global processes, which were in close agreement with those in the literature.
Palabras clave : Thermokinetics; pseudo-adiabatic calorimeter; zero; first and second order kinetics; simulations, H2O2 decomposition.













