Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Colombian Journal of Anestesiology
versión impresa ISSN 0120-3347
Rev. colomb. anestesiol. vol.40 no.2 Bogotá abr./jun. 2012
https://doi.org/10.1016/S0120-3347(12)70029-1
Review
http://dx.doi.org/10.1016/S0120-3347(12)70029-1
Hypocapnia in Neuroanesthesia: Current Situation
Hipocapnia en neuroanestesia: estado actual
María E. Solano C.a,*t Ichel Castillo B.b, María C. Niño de Mejíac
a Resident 111 year Anesthesiology, Universidad del Rosario, Bogotá, Colombia
b Resident 111 year Anesthesiology, Universidad de la Sabana, Bogotá, Colombia
c Neuroanesthesiology, 1ntensivist, Fundación Santa Fe de Bogotá, Bogotá, Colombia
ARTICLE INFO
Article history: Received: July 19, 2011 Accepted: February 18, 2012
ABSTRACT
Introduction: Hyperventilation has been a usual maneuver in the management of anesthesia in neurosurgical procedures. A few years back there used to be some medical skepticism about the potential of cerebral ischemia and today we know that it is detrimental and worsens the patient’s condition and prognosis.
Objective: To review the adverse effects of hypocapnia on various organs —mainly the brain— and to identify the current recommendations about its use.
Methodology: We conducted a PubMed literature search using MeSH terminology including the key words. The search was expanded to include a review of several texts and the bibliography of the most relevant articles.
Results: The literature review showed that hypocapnia is harmful for the brain and for other tissues and the current recommendation is to use it for two situations only: in case of imminent herniation and to improve the surgical field, limited to 20 minutes.
Conclusions: Hyperventilation should not be a routine anesthetic intervention for the management of the neurosurgical patient; there must be a precise indication and once the situation is corrected, the intervention must be immediately withdrawn.
Keywords: Hypocapnia Anesthesia Hyperventilation Carbon dioxide
© 2011 Sociedad Colombiana de Anestesiología y Reanimación. Published by Elsevier.
All rights reserved.
RESUMEN
Introducción: La hiperventilación ha sido una maniobra común en el manejo anestésico de procedimientos neuroquirúrgicos. Hace unos años había escepticismo entre los médicos sobre si esto resultaba en isquemia cerebral. Hoy sabemos que es perjudicial y deteriora el estado y el pronóstico del paciente.
Objetivo: Hacer una revisión de los efectos adversos de la hipocapnia en diferentes órganos, principalmente el cerebro, e identificar las recomendaciones actuales de su utilidad.
Métodos: Realizamos una búsqueda de la literatura en base de datos de PubMed utilizando términos MeSH incluidos en las palabras clave; se amplió con la revisión de algunos textos y la bibliografía de los artículos más relevantes.
Resultados: Con la revisión de la literatura, se ha demostrado que la hipocapnia es perjudicial tanto para el cerebro como para otros tejidos, y la recomendación actual es utilizarla sólo en dos situaciones (en caso de herniación inminente y para mejorar el campo quirúrgico) y por 20 min.
Conclusiones: La hiperventilación no debe ser una intervención anestésica rutinaria en el manejo del paciente neuroquirúrgico; debe tener una indicación precisa y, una vez la indicación haya cesado, la intervención debe ser retirada lo más pronto posible.
Palabras clave: Hipocapnia Anestesia Hiperventilación Dióxido de carbono
© 2011 Sociedad Colombiana de Anestesiología y Reanimación. Publicado por Elsevier.
Todos los derechos reservados.
Introduction
Hyperventilation has been a frequent manoeuver in the management of anesthesia in elective and emergency neurosurgical procedures.1-4 A few years back there used to be some skepticism about the potential development of cerebral ischemia;3,4 in healthy brains this may be harmless, but under pathological conditions it can be harmful and worsen the patients clinical condition and prognosis.1,4
The purpose of the article is to review the adverse effects of hypocapnia on the brain and other organs, and to identify the current recommendations for its use.
Material and methods
We conducted a PubMed literature search using MeSH terminology including the key words. The search was expanded to include a review of several texts and the bibliography of the most relevant article.
Metabolism and CO2 transport
CO2 is excreted from the cell into the interstitial fluid as the final outcome of the metabolic activity.5 CO2 is an extremely soluble gas transported in the blood: 5-10% dissolved, generating the CO2 arterial blood pressure (PaCO2), 20-30% bound to proteins and forming carbamine complexes and 65-70% as bicarbonate (HCO3-).1 This transport is complex and focuses on the CO2 -water reaction to produce carbonic acid (H2CO3),6 which maintains its equilibrium with H+ and HCO3- through a slow reaction that takes 40 seconds.1,6 This reaction progresses considerably inside the erythrocytes due to the presence of the carbonic anhydrase enzyme, and is completed in less than 10 milliseconds.6 H2CO3 dissociates into H+ and HCO3-, and a large bicarbonate fraction is pumped into the plasma and is exchanged by chloride; the hydrogen ions are buffered by hemoglobin6 (fig. 1).
PaCO2 represents the balance between production and elimination,1,2 and in healthy people this balance is within the physiological range.2
The following formula reflects such situation:
PaCO2 = production/elimination+inspired CO2
Since the inspired CO2 is insignificant and a drop in production is unusual, we concluded that any changes in PaCO2 are the direct consequence of its alveolar ventilation elimination.2
The critical patient may develop accidental hypocapnia as a consequence of mechanical ventilation2, but it can also be due to the pre hospital admission management of patients with trauma brain injury (TBI) in which the absolute values and duration of the hypocapnia are associated with adverse events.7 Intentionally induced hypocapnia can be seen in the management of increased intracranial pressure (ICP) patients or neonates with pulmonary arterial hypertension (PAH).1
In healthy individuals, hypocapnia (even if manifest) does not result in significant adverse events,2 and brain ischemia will not occur with a PaCO2 >20 mmHg.3 When symptoms are present, these include: paresthesia, palpitations, myalgia and seizures.2 Studies in human volunteers and healthy animals with severe hypocapnia (PaCO2 <15 mmHg), found metabolic and electroencephalographic disorders.3 One study found electroencephalographic alterations and paresthesia with PaCO2 <20 mmHg that could be reversed with the administration of hyperbaric oxygen, which suggests that the origin could be ischemic.3
Lundberg described hyperventilation in 1950 as a means to lower elevated ICP;4 hence then its usefulness as a therapeutic tool in patients with severe intracranial hypertension (ICH) has been historically accepted, particularly related to severe head injury,4 and neonates with PAH2 in whom the transient induction of hypocapnia leads to potentially life saving physiological changes. There have been some reports describing the reversal of the clinical signs of herniation such as fixed and dilated pupils, following aggressive hyperventilation.4 Just the opposite outcome results with prolonged hypocapnia in critical patients and is associated with poor prognosis and clinical results.8
Definition
Hypocapnia is defined as a PaCO2 <35 mmHg at sea level,5 and the reduced PaCo2 is the result of increased alveolar ventilation and is considered a synonym of hypocapnia.9
In terms of the severity classification,10 it must be highlighted that the normal PaCO2 levels should be determined based on the barometric pressure at varying altitudes.9
Trend towards the use of hyperventilation
Historically, hyperventilation has been considered beneficial to reduce the adverse effects of ICH;11 however, in 1991 Muizelaar reported poorer results in hyperventilated brain injured patients in whom the PaCO2 was maintained at 25 mmHg.7,11 Its use and safety in the management of ICH have been extensively debated for years,12,13 but is still a frequent approach for comatose patients with any type of brain injury in the ICU.7
Hypocapnia is widely used in adults and children with acute brain injury, even in early stages when ICH has not yet developed.10 Hypocapnia continues to be used by physicians and paramedics, despite the well-known deleterious effects and the existing guidelines recommending to avoid the use of this practice.14
Adult scenario
Neumann et al15 studied the arterial gasses in 2269 ventilation cases in TBI patients and found that early prophylactic hyperventilation in the first 24 hours was administered in 54% of the cases. Furthermore, most patients without intracranial hypertension exhibited significant hypocapnia over 50% of the total ventilation time.14,15 Unfortunately, the trend of neurosurgeons in the US is routine use of prophylactic hyperventilation to manage severe brain trauma patients in 36% of the cases.10,14
Children scenario
Hypocapnia is still widely used in pediatric patients with acute brain injury, despite the trauma guidelines published in 200316 advising against its use because of the undesirable effects in this population which is considered to be more susceptible to injury. An analysis by the Brain Trauma Foundation (BTF) found that 52% of the patients were hyperventilated and the most worrisome group is that of children under 2 years of age because of the higher incidence of severe hypocapnia and the risk of intraventricular bleeding.17 An article published by Rebecca Curry in 200916 studied the incidence of severe hypocapnia (PaCO2 <30 mmHg) in the pediatric population with TBI, after the publication of the guidelines, and concluded that although the PaCO2 measurement was more frequent and severe hypocapnia dropped, the incidence is still high during the 48 hours following the patient's admission and the time to measure PaCO2 was longer in younger children resulting in higher hypocapnia and became a predictor of mortality.
Pre-hospitalization scenario
About 50% of ER doctors in Michigan use prophylactic hyperventilation in brain injury patients as a routine but accidental hyperventilation is also frequent.18 This results in severe hypocapnia (expired CO2 <30 mmHg) in 70% of the patients transferred by helicopter, which means that the patient develops hypocapnia during the pre-hospitalization management and ICU admission, which further obscures the prognosis.10,18
Brain effect
Hypocapnia reduces the brain blood volume (BBV) as a direct consequence of a reduced cerebral blood flow (CBF), but the magnitude of the impact is limited.10 PET studies showed that only 30% of the CBF is found in the arteries.19
The potential drop in CBF is around 3% per mmHg modified in the PaCO2 within the 20-60 mmHg range,10 so that a 2025 mmHg reduction in the PaCO2 decreases the CBF by 40-50%.1
The effect on the venous vessels is minimal and dynamic PET studies showed that CO2 induced changes in CBV are due to alterations in the arterial volume but not in the arteriolar or venous volume.20 The net effect is a 30% reduction in CBF while CBV only drops 7%.10 Aiming at higher levels of hypocapnia results in further CBF impairment but no additional impact on the CBF or ICP. Hence, in order to achieve a considerable CBV reduction the CO2 must drop to levels that could compromise the CBF, which in severely ill brains sacrifices tissue perfusion.
There is a discussion going on regarding weather vasoreactivity is mediated by changes in CO2 or in pH. Recent studies favor the pH theory with effects on the smooth muscle, preferably in the smaller arteries (pial arterioles), considered to be more sensitive and subject to the involvement of several molecular mechanisms (nitric oxide, vasoactive prostanoids, ATP sensitive calcium channels and calcium channels).10,21,22
Hipoxia and hipocapnia induced cerebral ischemia
The injured areas exhibit increased CO2 vasoreactivity, which aggravates the ischemic injury because the flow is derived from the injured onto the normal areas. Furthermore, hypocapnic alkalosis causes bronchoconstriction and attenuates the hypoxic pulmonary vasoconstriction, leading to a decreased
PaO2.23
The oxyhemoglobin dissociation curve is altered and at any PaO2 moves to the left, with increased affinity for hemoglobin and hindering the delivery of O2 to the tisues.24
The cerebral ischemia may worsen with an increased O2 demand due to increased neuronal excitability.25,26 This contributes to a more extensive glucose utilization and depletion, changing over to anaerobic metabolism.27 Hypocapnia raises the oxygen metabolic rate in brain injury28 and prolongs the convulsive activity leading to the production of local excitatory, cytotoxic amino acids including N-metil-D-aspartate associated with seizures.29
Effect on the neonatal brain
Hyperventilation is deleterious to the premature brain, with white matter substance involvement and is considered
an isolated risk factor for periventricular leukomalacia, syndrome associated with significant neonatal mortality and neurodevelopmental deficit.30,31
Preterm babies exposed to short periods of severe hypocapnia (PaCO2 <15 mmHg) have been associated with long term neurological abnormalities such as neurosensory auditory loss.10 Some of the predisposing factors include: vulnerable areas of poor vascular development, antioxidants depletion due to excitatory amino acids and effect of sepsis induced cytokines and lipopolysaccharides, which drive the destruction of the white matter.10,32
The abrupt interruption of hyperventilation is associated to reactive hyperemia since normalization of PaCO2 causes cerebral vasodilation and intracranial hemorrhage in premature babies.2,33
Usefulness in brain injury
Brain injury is the most disabling injury in the United States with 2% of Americans currently living with secondary disabilities.8
The rationale for using hyperventilation in brain injured patients has a theoretical foundation based on the Monro-Kelly Doctrine, which states that the cranial compartment is incompressible, and the volume inside the cranium is a fixed volume. The cranium and its constituents create a state of volume equilibrium, such that any increase in volume of one of the cranial constituents must be compensated by a decrease in volume of another.10 We know that the cranial compartment is a closed space where its three main components brain tissue (80%), blood volume (5%), and CSF (15%) play a key role in homeostasis of the CBF and if one is increased without compensating with change in another, or when the compensation threshold is exceeded, an abrupt elevation in ICP occurs leading to decreased CBF.10
The purpose of inducing hypocapnia is to reduce the ICP by generating cerebral arterial hypocapnia and decreased CBV10 (fig. 2).
Cerebral edema develops in 40% of severe TBI patients and ICH is one of the main causes of death and neurological disability.34 However, the mechanism of ICP reduction is not physiologically harmless; during the first 24 hours post-trauma there is a decrease in the CBF, exposing the brain to ischemia secondary to aggressive hyperventilation.10,11 Studies of post-traumatic CBF indicate that during the first 4-12 hours CBF drops to one half of the normal level.35 Bouman et al36 found that within the first 3 hours of the injury, the global/ regional CBF was <18 ml/100 g/min in 31% of the patients, which is under the "ischemic threshold". This abnormally low flow was most frequent in the brain tissue within or around the hemorrhagic lesion and underlying the acute subdural hematoma.37 The transcranial doppler evidenced an early slow CBF in up to two thirds of these patients.36,38
Additionally, post-mortem studies by Graham et al39, found ischemic changes in around 90% of the patients who died from TBI.
A study by Muizelaar et al8 in patients with moderate TBI included both a normocapnic and hypocapnic group maintaining the PaCO2 at 35 mmHg and 25 mmHg respectively, and the results at 3 and 6 months following the injury revealed poor results for the hyperventilated group. Centers that monitor SvjO2 have observed the potential to raise low values and the mean lactate in the jugular vein can be decreased by reducing the level of hyperventilation.40,41
a process that may develop within minutes and last from several hours to a few days. Hence the CSF pH is normalized and the CBF returns to normal levels within a 6 hour period even if the PaCO2 remains depresed.10 Evidence from four decades ago in healthy volunteers showed that a sustained reduction of 20 mmHg in PaCO2 immediately achieved a 40% reduction in CBF and after 4 hours the CBF had recovered to 90% of its normal value.42
The rebound reaction of ICP following the recovery of normocapnia has been well described and this is due to the fact that a PaCO2 increase reduces the CSF pH that had been normalized through buffering. This respiratory acidosis results in an increased CBF, which results in rebound hyperemia10 (fig. 3).
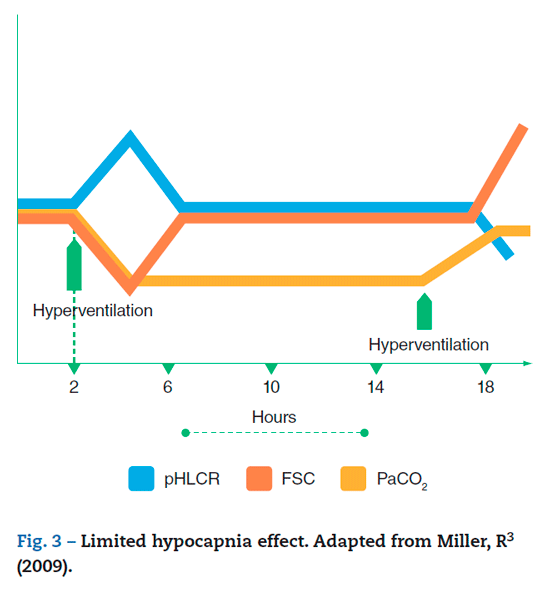
Limited effect of hypocapnia
The effect of hypocapnia on the CBF is not sustained and is progressively lost over time, due to the loss of effect over the ICP.3 Once the hyperventilation sets in, the resulting hypocapnia and respiratory acidosis rapidly raise the CSF pH and the extracellular fluid in the CNS with a subsequent drop in the CBF. In prolonged hypocapnia, the effect is limited by a buffering effect that enables the CSF pH to return to normal levels, and hence the CBF is normalized. Buffering is a biphasic process; first, the alkalosis derived from hypocapnia stimulates the release of chloride ions from the intracellular into the extracellular fluid with bicarbonate exchange; this mechanism is called tissue buffer and occurs almost immediately. Secondly, there is a renal response resulting from the inhibition of tubular reabsorption of bicarbonate and the secretion of hydrogen ions at the proximal tubule,
Brain Trauma Foundation recommendations
The BTF43 believes that there are insufficient data in the literature for a level I recommendation of hyperventilation in TBI.
Prophylactic hyperventilation (PaCO2 <25 mmHg) is not recommended in TBI. Studies measuring the CBF using xenon and thermodiffusion,35,36,44 evidenced that CBF is dangerously decreased following TBI for up to 48 hours. Level II Evidence.
Hyperventilation is recommended as a temporary measure to lower elevated ICP. Level III recommendation.
The suggestion is that hyperventilation should be avoided during the first 48 hours following TBI since at that point the CBF is usually critically diminished and the recommendation is to take SvjO2 and brain tissue oxygen pressure (PbrO2) measurements, aimed at monitoring the oxygen delivery. Consequently, limiting the use of hyperventilation following
severe TBI may help with neurological recovery after trauma, or at least avoid iatrogenic cerebral ischemia.
Other effects of hypocapnia
Most systemic effects of hyperventilation result from decreased PaCO2 alkalosis, and this has been documented in the brain, the lungs, the heart, the placenta and the peripheral tissues.45
Postoperative psychomotor dysfunction
The potential of hypocapnia (even if short lasting) to cause cognitive impairment is clearly document during the postoperative period. Healthy patients undergoing hypocapnia express an impaired cognitive function for up to 48 hours and this is more evident in the elderly who are more susceptible and vulnerable to its consequences, even with less severe hypocapnia.2,10 Furthermore, hypocapnia may impair attention, learning and trigger personality changes which although long-lasting, are reversible.46
Lung
Hypocapnia raises the airway resistance due to bronchospasm and increases the permeability in the microvasculature.2 Over 20% of TBI patients exhibit acute pulmonary injury and develop adult respiratory distress syndrome (ARDS), which worsens the prognosis. Unfortunately, some of these patients continue to be hyperventilated despite the known association between an elevated tidal volume and ARDS mortality.10
Hypocapnia attenuates hypoxic pulmonary vasoconstriction worsening the intrapulmonary shunt and causing impaired systemic oxygenation.2 Additionally, it contributes to acute pulmonary injury because a high tidal volume directly injures the lung.10,47
Cardiovascular system
Cardiovascular effects mainly involve myocardial oxygenation disorders and heart rate disorders.2
Myocardial ischemia. Acute hypocapnia reduces the oxygen delivery while increasing the demand. The latter is due to several mechanisms, including increased myocardial contractility and systemic vascular resistance).2,10 It has also been shown to facilitate thrombosis due to a raise in the number of platelets and platelet aggregation. As a result of these effects there is overt coronary spasm resulting in variant angina, which usually presents with spontaneous hyperventilation.48
Cardiac arrhythmias. Hypocapnia is associated with the development of arrhythmia, both in critical patients and in panic attacks,3 including paroxysmal atrial arrhythmias
and very seldom, ventricular tachycardia or ventricular fibrilation.10 The effects are secondary to ischemia but are also associated with a direct myocardial effect.
The placenta and the fetus. In pregnancy, PaCO2 is maintained 10 mmHg below the normal values. This physiological status is accompanied by a decreased bicarbonate concentration and is rapidly normalized after delivery. However, a greater decrease, even for short periods of time, could result in significant adverse events for the fetus (decreased fetal PaO2, increased baseline deficit, low Apgar score and delayed onset of rhythmic breathing).49 Hypocapnia associated alkalosis decreases placental perfusion, reduces oxygen pressure in the umbilical vein and stimulates a reflex spasm of the umbilical vein.2,49
Current use of hypocapnia in acute brain injury
Imminent brain herniation. There is a b physiological reason for the short use of hypocapnia to acutely reduce ICP. Although the evidence is limited, the fast induction and its immediate effect on the CBF make it a useful strategy while more definite measures are established.
Intraoperative use in neurosurgery. In this situation hypocapnia serves to facilitate the surgical access and acutely reduce the cerebral edema. This was shown in a randomized, prospective study in patients with supratentorial tumors in which 20 minutes of hyperventilation managed to reduce the cerebral edema and the ICP.50
Once the acute hypocapnia sets in, normocapnia must be established as soon as possible since it looses effectiveness and could be harmful by causing rebound hyperemia when the PaCO2 becomes normal.10
Conclusions
Currently the recommendation to use hypocapnia is limited to special cases since its deleterious effect have been proven not only for the brain, but also for other organs where perfusion is compromised. Hyperventilation should not be a routine maneuver in anesthesia and much less in neuroanesthesia; the current guidelines advise against prophylactic hyperventilation and the available evidence indicates that it may result in schemia, particularly when the basal CBF is diminished as is the case in the 24 hours following TBI.
The more severe and the longer the duration of hypocapnia, the higher the possibility of injury. Therefore, using mild to moderate hypocapnia for short time intervals (20 minutes), limits the damage and reduces the possibility of developing rebound hyperemia, and avoids missing on the possibility to use it as a salvage procedure when needed.
Just as any other therapeutic intervention, there has to be a clear indication for hypocapnia (elevated ICP with imminent herniation and/or the need to improve the conditions in the surgical field). Hence, whenever using pypocapnia, keep in mind its potentially harmful effects, use it for short time
intervals while definite measures are adopted for controlling the ICP and as soon as the indication ceases, proceed to normalize the PaCO2 promptly.
Funding
Authors' own resources.
Conflict of interests
None declared.
REFERENCES
1. Curley G, Laffey J, Kavanagh B. Bench-to-beside review: Carbon dioxide. Crit Care. 2010;14:220.
2. Laffey J, Kavanagh B. Hypocapnia. N Engl J Med. 2002.
3. Miller R. Miller's Anesthesia. 7.a ed. 2009.
4. Marion D, Puccio A, Wisniewski S. Effect of hyperventilation on extracellular concentrations of glutamate, lactate, and local cerebral blood flow in patients with severe traumatic brain injury. Crit Care Med. 2002;30:2619-25.
5. Patiño F, Celis E. Fisiología de la respiración e insuficiencia respiratoria aguda. 7.a ed. 2005.
6. Marino P. The UCI book. 3.a ed. Capítulo 2. 2007.
7. Ghajar J, Hariri R, Narayan R. Survey of critical care management of comatose, head-injured patients in the United States. Crit Care Med. 1995;23:560-7.
8. Muizelaar JP, Marmarou A, Ward JD. Adverse effects of prolonged hyperventilation in patients with severe head injury. J Neurosurg. 1991;75:731-9.
9. Stocchetti N, Maas A, Chieregato A. Hyperventilation in head injury. Chest. 2005;127:1812-27.
10. Curley G, Kavanagh B, Laffey J. Hypocapnia and the injured brain: More harm than benefit. Crit Care Med. 2010.
11. Caulfield E, Dutton R, Floccare D. Prehospital hypocapnia and poor outcome after severe traumatic brain injury. J Trauma. 2009;66:1577-83.
12. Carmona Suazo JA, Maas AIR, Van den Brink. CO2 reactivity and brain oxygen pressure monitoring in severe head injury. Crit Care Med. 2000;28:3268-74.
13. Skippen P, Seear M, Poskitt K. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25:1402-9.
14. Marion DW, Spiegel TP. Changes in the management of severe traumatic brain injury: 1991-1997. Crit Care Med. 2000;28:16-8.
15. Neumann JO, Chambers IR, Citerio G. The use of hyperventilation therapy after traumatic brain injury in Europe. Intensive Care Med. 2008;34:1676-82.
16. Curry R, Hollingworth W, Richard G. Incidence of hypo- and hypercarbia in severe traumatic brain injury before and after 2003 pediatric guidelines. Pediatr Crit Care Med. 2008;9:141-6.
17. Brain Trauma Foundation. Use of hyperventilation in the acute management of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:45-7.
18. Huizenga JE, Zink B, Maio R. The penetrance of head injury management guidelines into the practice patterns of Michigan emergency physicians. Acad Emerg Med. 2000;7:1171.
19. Ito H, Kanno I, Iida H. Arterial fraction of cerebral blood volume in humans measured by positron emission tomography. Ann Nucl Med. 2001;15:111-6.
20. Ito H, Ibaraki Kanno I, Fukuda H, Miura S. Changes in the arterial fraction of human cerebral blood volume during hypercapnia and hipocapnia measured by positron emission tomography. J Cereb Blood Flow Metab 2005;25:852-857.
21. Kontos HA, Raper AJ, Patterson JL. Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke. 1977;8:358-60.
22. Ozan A. Optimizing the intraoperative management of carbon dioxide concentration. Curr Opin Anaesthesiol. 2006;19:19-25.
23. Domino KB, Emery MJ, Swenson ER, et al. Ventilation heterogeneity is increased in hypocapnic dogs. Respir Physiol. 1998;111:89-100.
24. Nunn JF. Applied respiratory physiology. 3.a ed. London: Butterworths; 1987.
25. Huttunen J, Tolvanen H, Heinonen E, et al. Effects of voluntary hyperventilation on cortical sensoryresponses. Electroencephalographic and magnetoencephalographic studies. Exp Brain Res. 1999;125:248-54.
26. Davis D. Early ventilation in traumatic brain injury. Resuscitation. 2008;76:333-40.
27. Samra SK, Turk P, Arens JF. Effect of hipocapnia on local cerebral glucose utilization in rats. Anesthesiology. 1989;70:523-6.
28. Coles JP, Fryer TD, Coleman MR, et al. Hyperventilation following head injury: Effect on ischemic burden and cerebral oxidative metabolism. Crit Care Med. 2007;35:568-78.
29. Graham EM, Apostolou M, Mishra OP, Delivoria-Papadopoulos M. Modification of the N-methyl-D-aspartate receptor in the brain of newborn piglets following hyperventilation induced ischemia. Neurosci Lett. 1996;218:29-32.
30. Ikonen RS, Janas MO, Koivikko MJ, Laippala P, Kuusinen EJ. Hyperbilirubinemia, hypocarbia and periventricular leukomalacia in preterm infants. Acta Paediatr. 1992;81:802-7.
31. Wiswell TE, Graziani LJ, Kornhauser MS, Stanley C, Merton DA, McKee L, et al. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high frequency jet ventilation. Pediatrics. 1996;98:918-24.
32. Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, et al. High expression of tumour necrosis factor a and interleukin6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406-11.
33. Gleason CA, Short BL, Jones D. Cerebral blood flow and metabolism during and after prolonged hypocapnia in newborn lambs. J Pediatr. 1989;115:309-14.
34. Narayan RK, Kishore PRS, Becker DP, Ward JD, Enas GG, Greenberg RP, et al. Intracranial pressure: to monitor or not to monitor. J Neurosurg. 1982;56:650-9.
35. Marion DW, Darby J, Yonas H. Acute regional cerebral blood flow changes caused by severe head injuries. J Neurosurg. 1991;74:407-14.
36. Bouma GJ, Muizelaar JP, Stringer WA, Choi SC, Fatouros P, Young HF. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J Neurosurg. 1992;77:360-8.
37. Heiss WD, Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol. 1983;14:294-301.
38. Van Santbrink H, Schouten JW, Steyerberg EW, et al. Serial transcranial Doppler measurements in traumatic brain injury with special focus on the early posttraumatic period. Acta Neurochir. 2002;144:1141-9.
39. Graham DI, Lawrence AE, Scott G, et al. Brain damage in fatal non-missile head injury without high intracranial pressure. J Clin Pathol. 1988;41:34-7.
40. Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. Cerebral circulation and metabolism after severe traumatic brain injury. J Neurosurg. 1991;75:685-93.
41. Gopinath SP, Robertson CS, Contant CF, Hayes C, Feldman Z, Narayan RK, et al. Jugular venous desaturation and outcome after head injury. J Neurol Neurosurg Psychiatry. 1994;57:717-23.
42. Raichle ME, Posner JB, Plum F. Cerebral blood flow during and after hyperventilation. Arch Neurol. 1970;23:394-403.
43. Guidelines for the Management of Severe Traumatic Brain Injury 2007. A Joint project of the Brain Trauma Foundation. Journal of Neurotrauma.
44. Sioutos PJ, Orozco JA, Carter LP, Weinand ME, Hamilton AJ, Williams FC. Continuous regional cerebral cortical blood flow monitoring in head-injured patients. Neurosurgery. 1995;36:943-9.
45. Newell DW, Weber JP, Watson R, Aaslid R, Winn HR. Effect of transient moderate hyperventilation on dynamic cerebral autoregulation after severe head injury. Neurosurgery. 1996;39:35-44.
46. Hovorka J. Carbon dioxide homeostasis and recovery after general anaesthesia. Acta Anaesthesiol Scand. 1982;26: 498-504.
47. Laffey JG, Engelberts D, Duggan M, Veldhuizen R, Lewis JF, Kavanagh B. Carbon dioxide attenuates pulmonary impairment resulting from hyperventilation. Crit Care Med. 2003;31:2634-40.
48. Hisano K, Matsuguchi T, Ootsubo H, Nakagaki O, Tomoike H, et al. Hyperventilation-induced variant angina with ventricular tachycardia. Am Heart J. 1984;108:423-5.
49. Cook PT. The influence on foetal outcome of maternal carbon dioxide tension at caesarean section under general anaesthesia. Anaesth Intens Care. 1984;12: 296-302.
50. Gelb AW, Craen RA, Rao GS, Reddy KRM, Megyesi J,Mohanty B, et al. Does hyperventilation improve operating condition during supratentorial craniotomy? Anesth Analg. 2008;106:585-94.
1. Curley G, Laffey J, Kavanagh B. Bench-to-beside review: Carbon dioxide. Crit Care. 2010;14:220. [ Links ]
2. Laffey J, Kavanagh B. Hypocapnia. N Engl J Med. 2002. [ Links ]
3. Miller R. Miller's Anesthesia. 7.a ed. 2009. [ Links ]
4. Marion D, Puccio A, Wisniewski S. Effect of hyperventilation on extracellular concentrations of glutamate, lactate, and local cerebral blood flow in patients with severe traumatic brain injury. Crit Care Med. 2002;30:2619-25. [ Links ]
5. Patiño F, Celis E. Fisiología de la respiración e insuficiencia respiratoria aguda. 7.a ed. 2005. [ Links ]
6. Marino P. The UCI book. 3.a ed. Capítulo 2. 2007. [ Links ]
7. Ghajar J, Hariri R, Narayan R. Survey of critical care management of comatose, head-injured patients in the United States. Crit Care Med. 1995;23:560-7. [ Links ]
8. Muizelaar JP, Marmarou A, Ward JD. Adverse effects of prolonged hyperventilation in patients with severe head injury. J Neurosurg. 1991;75:731-9. [ Links ]
9. Stocchetti N, Maas A, Chieregato A. Hyperventilation in head injury. Chest. 2005;127:1812-27. [ Links ]
10. Curley G, Kavanagh B, Laffey J. Hypocapnia and the injured brain: More harm than benefit. Crit Care Med. 2010. [ Links ]
11. Caulfield E, Dutton R, Floccare D. Prehospital hypocapnia and poor outcome after severe traumatic brain injury. J Trauma. 2009;66:1577-83. [ Links ]
12. Carmona Suazo JA, Maas AIR, Van den Brink. CO2 reactivity and brain oxygen pressure monitoring in severe head injury. Crit Care Med. 2000;28:3268-74. [ Links ]
13. Skippen P, Seear M, Poskitt K. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25:1402-9. [ Links ]
14. Marion DW, Spiegel TP. Changes in the management of severe traumatic brain injury: 1991-1997. Crit Care Med. 2000;28:16-8. [ Links ]
15. Neumann JO, Chambers IR, Citerio G. The use of hyperventilation therapy after traumatic brain injury in Europe. Intensive Care Med. 2008;34:1676-82. [ Links ]
16. Curry R, Hollingworth W, Richard G. Incidence of hypo- and hypercarbia in severe traumatic brain injury before and after 2003 pediatric guidelines. Pediatr Crit Care Med. 2008;9:141-6. [ Links ]
17. Brain Trauma Foundation. Use of hyperventilation in the acute management of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:45-7. [ Links ]
18. Huizenga JE, Zink B, Maio R. The penetrance of head injury management guidelines into the practice patterns of Michigan emergency physicians. Acad Emerg Med. 2000;7:1171. [ Links ]
19. Ito H, Kanno I, Iida H. Arterial fraction of cerebral blood volume in humans measured by positron emission tomography. Ann Nucl Med. 2001;15:111-6. [ Links ]
20. Ito H, Ibaraki Kanno I, Fukuda H, Miura S. Changes in the arterial fraction of human cerebral blood volume during hypercapnia and hipocapnia measured by positron emission tomography. J Cereb Blood Flow Metab 2005;25:852-857. [ Links ]
21. Kontos HA, Raper AJ, Patterson JL. Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke. 1977;8:358-60. [ Links ]
22. Ozan A. Optimizing the intraoperative management of carbon dioxide concentration. Curr Opin Anaesthesiol. 2006;19:19-25. [ Links ]
23. Domino KB, Emery MJ, Swenson ER, et al. Ventilation heterogeneity is increased in hypocapnic dogs. Respir Physiol. 1998;111:89-100. [ Links ]
24. Nunn JF. Applied respiratory physiology. 3.a ed. London: Butterworths; 1987. [ Links ]
25. Huttunen J, Tolvanen H, Heinonen E, et al. Effects of voluntary hyperventilation on cortical sensoryresponses. Electroencephalographic and magnetoencephalographic studies. Exp Brain Res. 1999;125:248-54. [ Links ]
26. Davis D. Early ventilation in traumatic brain injury. Resuscitation. 2008;76:333-40. [ Links ]
27. Samra SK, Turk P, Arens JF. Effect of hipocapnia on local cerebral glucose utilization in rats. Anesthesiology. 1989;70:523-6. [ Links ]
28. Coles JP, Fryer TD, Coleman MR, et al. Hyperventilation following head injury: Effect on ischemic burden and cerebral oxidative metabolism. Crit Care Med. 2007;35:568-78. [ Links ]
29. Graham EM, Apostolou M, Mishra OP, Delivoria-Papadopoulos M. Modification of the N-methyl-D-aspartate receptor in the brain of newborn piglets following hyperventilation induced ischemia. Neurosci Lett. 1996;218:29-32. [ Links ]
30. Ikonen RS, Janas MO, Koivikko MJ, Laippala P, Kuusinen EJ. Hyperbilirubinemia, hypocarbia and periventricular leukomalacia in preterm infants. Acta Paediatr. 1992;81:802-7. [ Links ]
31. Wiswell TE, Graziani LJ, Kornhauser MS, Stanley C, Merton DA, McKee L, et al. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high frequency jet ventilation. Pediatrics. 1996;98:918-24. [ Links ]
32. Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, et al. High expression of tumour necrosis factor a and interleukin6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406-11. [ Links ]
33. Gleason CA, Short BL, Jones D. Cerebral blood flow and metabolism during and after prolonged hypocapnia in newborn lambs. J Pediatr. 1989;115:309-14. [ Links ]
34. Narayan RK, Kishore PRS, Becker DP, Ward JD, Enas GG, Greenberg RP, et al. Intracranial pressure: to monitor or not to monitor. J Neurosurg. 1982;56:650-9. [ Links ]
35. Marion DW, Darby J, Yonas H. Acute regional cerebral blood flow changes caused by severe head injuries. J Neurosurg. 1991;74:407-14. [ Links ]
36. Bouma GJ, Muizelaar JP, Stringer WA, Choi SC, Fatouros P, Young HF. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J Neurosurg. 1992;77:360-8. [ Links ]
37. Heiss WD, Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol. 1983;14:294-301. [ Links ]
38. Van Santbrink H, Schouten JW, Steyerberg EW, et al. Serial transcranial Doppler measurements in traumatic brain injury with special focus on the early posttraumatic period. Acta Neurochir. 2002;144:1141-9. [ Links ]
39. Graham DI, Lawrence AE, Scott G, et al. Brain damage in fatal non-missile head injury without high intracranial pressure. J Clin Pathol. 1988;41:34-7. [ Links ]
40. Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. Cerebral circulation and metabolism after severe traumatic brain injury. J Neurosurg. 1991;75:685-93. [ Links ]
41. Gopinath SP, Robertson CS, Contant CF, Hayes C, Feldman Z, Narayan RK, et al. Jugular venous desaturation and outcome after head injury. J Neurol Neurosurg Psychiatry. 1994;57:717-23. [ Links ]
42. Raichle ME, Posner JB, Plum F. Cerebral blood flow during and after hyperventilation. Arch Neurol. 1970;23:394-403. [ Links ]
43. Guidelines for the Management of Severe Traumatic Brain Injury 2007. A Joint project of the Brain Trauma Foundation. Journal of Neurotrauma. [ Links ]
44. Sioutos PJ, Orozco JA, Carter LP, Weinand ME, Hamilton AJ, Williams FC. Continuous regional cerebral cortical blood flow monitoring in head-injured patients. Neurosurgery. 1995;36:943-9. [ Links ]
45. Newell DW, Weber JP, Watson R, Aaslid R, Winn HR. Effect of transient moderate hyperventilation on dynamic cerebral autoregulation after severe head injury. Neurosurgery. 1996;39:35-44. [ Links ]
46. Hovorka J. Carbon dioxide homeostasis and recovery after general anaesthesia. Acta Anaesthesiol Scand. 1982;26: 498-504. [ Links ]
47. Laffey JG, Engelberts D, Duggan M, Veldhuizen R, Lewis JF, Kavanagh B. Carbon dioxide attenuates pulmonary impairment resulting from hyperventilation. Crit Care Med. 2003;31:2634-40. [ Links ]
48. Hisano K, Matsuguchi T, Ootsubo H, Nakagaki O, Tomoike H, et al. Hyperventilation-induced variant angina with ventricular tachycardia. Am Heart J. 1984;108:423-5. [ Links ]
49. Cook PT. The influence on foetal outcome of maternal carbon dioxide tension at caesarean section under general anaesthesia. Anaesth Intens Care. 1984;12: 296-302. [ Links ]
50. Gelb AW, Craen RA, Rao GS, Reddy KRM, Megyesi J, Mohanty B, et al. Does hyperventilation improve operating condition during supratentorial craniotomy? Anesth Analg. 2008;106:585-94. [ Links ]











 texto en
texto en 

