Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Colombian Journal of Anestesiology
versión impresa ISSN 0120-3347
Rev. colomb. anestesiol. vol.43 no.3 Bogotá jul./sep. 2015
Case report
Anaesthesia and analgesia for bladder exstrophy correction. Case reports*
Anestesia y Analgesia para corrección de extrofia vesical. Reporte de tres casos
Leonardo PaIacios-Palaciosa,b,*, Kelly Jóhana Solazar-Ramírezc
a Anesthesiology, Instituto de Ortopedia Infantil Rooseuelt, Specialist in Anaesthesia and Cardiopulmonary Resuscitation, Universidad Nacional de Colombia, Bogotá, Colombia
b Masters in Pain Treatment, Universidad de Salamanca, Masters in Ultrasound-Guided Regional Anaesthesia, Universidad de Salamanca, Salamanca, España
c Third-Year Resident of Anaesthesiology and Resuscitation, Uniuersidad de la Sabana, Chía, Colombia
* Please cite this article as: Palacios-Palacios L, Salazar-Ramírez KJ. Anestesia y Analgesia para corrección de extrofia vesical. Reporte de tres casos. Rev Colomb Anestesiol. 2015;43:254-258.
** Corresponding author at: Carrera 20 # 88-32 apto. 603, Bogotá, Colombia.
E-mail address: palaciosleonardo@gmail.com (L.P. Palacios).
Article info
Article history: Received 11 November 2014 Accepted 2 March 2015 Available online 13 May 2015
Abstract
Introduction: Neonatal bladder exstrophy repairs imply correcting the genitourinary malformation, and closing and stabilizing the pelvic girdle with external fixation and traction. Successful results are achieved in terms of reduced urinary incontinence, adequate aesthetic appearance, improved quality of life, reduction of the risk associated with neonatal surgery and minimization of the number of procedures associated with multistage repairs. In such procedures, prolonged perioperative anaesthesia is key for the patient to tolerate the traction and external fixation, to help osteotomy healing, and to reduce tension in the surgical wound. Patients' age and weight have an effect on the risk of toxicity from local anaesthetics and respiratory depression from opioid analgesics. The prolonged use of caudal catheters in the management of these cases is associated with infection at the insertion site.
Case presentation: The article presents the cases of three infants between 7 months and 1 year of age taken to bladder exstrophy repair and pelvic osteotomy with tunnelled caudal catheter and continuous local anaesthetic infusion as perioperative anaesthetic management technique. The use of these techniques was aimed at reducing the risk of infection at the insertion site and the risks associated with prolonged pain management in infants.
Conclusion: The cases suggest that tunnelled caudal catheter placement and continuous local anaesthetic infusion are safe techniques in the management of prolonged anaesthesia in infants, decreasing the risk of insertion site infection.
Keywords: Anesthesia, caudal Analgesia, Bladder exstrophy Infant, Anesthesia, local.
Resumen
Introducción: La corrección de la extrofia vesical en el lactante menor implica la reparación de la malformación genitourinaria y el cierre y estabilización del anillo pélvico utilizando un tutor externo y tracción. Se obtienen resultados exitosos en la continencia urinaria del paciente, adecuado aspecto estético y buena calidad de vida reduciendo el riesgo que implica la cirugía neonatal y minimizando el número de procedimientos. La analgesia postoperatoria prolongada es fundamental para tolerar el tutor y/o la tracción, permitir la cicatrización de las osteotomías y reducir la tensión sobre la herida quirúrgica. La edad y el peso de estos pacientes aumentan el riesgo de toxicidad por anestésico local y de depresión respiratoria con el uso de opioides, al igual que el uso prolongado de catéteres caudales se asocia a infección del sitio de inserción.
Presentación de caso: Se describen tres casos de lactantes de 7 meses a 1 ano de edad llevados a corrección de extrofia vesical y osteotomía pélvica mas fijación con tutor externo donde el manejo analgésico postoperatorio se realizó mediante la infusión de anestésico local por catéter caudal tunelizado para prolongar el tiempo de analgesia y reducir el riesgo de infección.
Conclusión: Se señala el uso de la tunelización de los catéteres caudales y la infusión continua de anestésico local como técnica segura para el manejo analgésico prologado en el paciente lactante con reducción del riesgo de infección del sitio de inserción.
Palabras clave: Anestesia caudal, Analgesia, Extrofia de la vejiga, Lactante, Anestesia local.
Introduction
Bladder exstrophy is an infrequent congenital malformation with an incidence of 3.3 cases for every 100 000 live births and a 2.3/1 male-to-female ratio1.
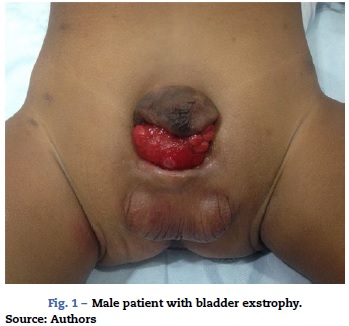
This abnormality consists of a congenital lower abdominal midline defect including symphysis pubis separation and abdominal wall defects such as bladder and urethral exposure, with a more anterior position of the bladder (Fig. 1). The finding of epispadias is quite frequent, although it is also possible to find cloacal involvement1,2. The surgical correction includes the closure of the bladder and the abdominal wall, together with approximation of the pelvis rami by means of osteotomy and external fixation.
Early primary closure of bladder and penis, with or without pelvic osteotomy, may be performed in the neonatal period, in infancy or later in childhood. In the newborn, the main considerations are the risks of major surgery and the anatomical and physiological limitations. When performed in infancy, the risk is lower after some months of weight gain and increased maturity. From childhood to the adult years, closure is performed late in several stages or if there is a history of failed closure. The main advantage of early closure is the potential for urinary continence, with renal function preservation and cosmetic and functional external genitalia2,3. In neonates, pelvic compliance sometimes allows manual approximation, although pelvic osteotomy is needed many times; however, external fixation is indicated in both cases in order to maintain pelvic closure for 4-6 weeks. Despite immobilization, results may be unfavourable, mainly in patients with pain and who are capable of pelvis elevation and balancing3.
Adequate prolonged postoperative analgesic management is critical in these patients in order to achieve a successful outcome1-4. Opioid analgesia is of limited use because of the high risk of respiratory depression and other adverse effects; epidural or caudal infusion of the local anaesthetic is associated with a risk of toxicity because of long-term requirement, and caudal catheter use increases the possibility of neuroaxial infection due to the proximity of the insertion site to the anus. In contrast, the potential motor block achieved with the epidural infusion of local anaesthetic is ideal for postoperative management.
Clinical case 1
Eight-month-old male patient, ASA II, weighing 8.2 kg, with a diagnosis of bladder exstrophy and pelvic diastasis and epispadias had normal term delivery, haematocrit 34.2%, and normal cardiopulmonary auscultation. Inhaled induction is performed with sevoflourane, canalization with a 22 G venous catheter and a right internal central jugular catheter, right radial arterial line, esophageal thermometer, caudal catheter inserted through an 18 G intravenous catheter and tunnelized with an 18 G, 80 mm Tuohy needle. Bladder exstrophy and epis-padias were corrected, with ureteroplasty using free mucosal graft, ureteroneocystostomy, bilateral pelvic osteotomy and external fixation. Intraoperative blood loss was 43% of the blood volume, requiring transfusion. The patient was transferred to the PICU, intubated, without haemodynamic support; caudal catheter analgesia with 0.075% levobupivacaine at a rate of 2.5cc/h (0,22mg/kg/h) was given, with adequate pain control and no signs of toxicity from the local anaesthetic or insertion site infection, and dipirone 20 mg/k IV at every 6 h. The patient developed Candida albicans urinary tract infection, mild pulmonary hypertension, nutritional decline and the need for mechanical ventilation and sedation. For this reason, the tunnelled catheter was removed on the third postoperative day and the patient was switched to fentanyl IV for sedation and analgesia. The patient evolved satisfactorily and was extubated on day 9. Oral feeding was started on day 11. The external pelvic fixation was removed after 66 days.
Clinical case 2
Seven-month-old male patient, ASA II, weighing 8.5 kg, with a diagnosis of bladder exstrophy, pelvic diastasis, epispadias and bilateral cryptorchidism had delivered normally at 38 weeks. Inhaled induction was with sevoflurane, with left radial arterial line, accessed with 22 g venous catheter in the right upper limb, and with left central external jugular vein catheter and caudal catheter tunnelling with tuohy 18. Bladder exstrophy correction, bilateral pelvic osteotomy, external fixation, epispadias correction, and orchidopexy were performed. Intraoperative blood loss was of 34% of the blood volume, requiring transfusion. Epidural infusion of 0.1% bupivacaine was at a rate of 2cc/h (0,23mg/kg/h), and dipirone 20mg/kg IV was for every 6 h. The patient evolved satisfactorily in the PICU and was extubated on day 2. Adequate pain management was followed. On the third postoperative day, the catheter became displaced in the PICU and was removed. Analgesia was continued with morphine 50mcg/kg IV every 4h. Oral intake was started on day 5, and the patient was transferred to the ward. The pelvic external fixator was removed after 44 days.
Clinical case 3
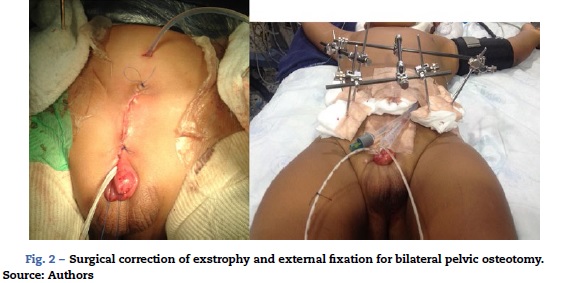
One-year-old male patient, ASA I/VI, weighing 13 kg, with a diagnosis of bladder exstrophy and epispadias, scheduled for exstrophy correction, bilateral pelvic osteotomy and external fixator placement had inhaled induction with sevoflurane, balanced general anaesthesia, access with #20 venous catheter in the right upper limb, central left external jugular vein catheter, right radial arterial line, analgesia with caudal tunnelled catheter (Fig. 2), and oesophageal temperature monitoring. Blood loss was of 270 cc (30% of the blood volume), requiring transfusion. The patient was haemodynamically stable in acid-base balance. The patient was transferred to the PICU, intubated, sedated and relaxed in order to reduce the risk of the urge to push. The patient was extubated on day 6. Postoperative analgesia was with 0.1% bupivacaine at a rate of 3.2cc/h (0.24 mg/kg/h) and dipirone 20mg/kg every 6h. The patient exhibited opioid withdrawal, which was managed with methadone 1 mg PO every 12 h. The epidural 0.2% bupivacaine infusion at 3cc/h (0.22 mg/kg/h) was maintained for 14 days and then switched to 0.1% lidocaine at 3 cc/h (0.45 mg/kg/h) for an additional 10 days. The catheter was removed after 23 days and management was continued with dipirone 15 mg/kg every 6 h and tramadol 1 mg/kg every 8 h. The patient was scheduled for fixator removal after completing 6 weeks of treatment.
Discussion
The fundamental objectives of bladder exstrophy correction are bladder closure, renal function preservation, adequate urinary continence, and adequate external genitalia cosmesis. There are hurdles to the achievement of these results, such as suture dehiscence, bladder prolapse, and multiple attempts at bladder closure. The approximation of the pelvic bones through the use of an external fixator reduces tension on the recently repaired midline, but pelvic osteotomies lead to higher blood loss and postoperative pain (Fig. 2)2,4-6.
In the first two cases described the tunnelled caudal catheter was left in place for a shorter period than planned, but it provided longer analgesia than usual. The first patient received continuous infusion for 3 days, but it was interrupted and the catheter removed because of haemodynamic instability, the need for high sedation to maintain mechanical ventilation, and the limitation in determining motor block. In the second patient, the catheter was inadvertently dislodged, but there was no pain during the time when the anaesthetic infusion was maintained. In the third patient, pain control was adequate for more than 3 weeks, starting with 0.1% bupi-vacaine followed by 0.2% lidocaine after 2 weeks in order to reduce the risk of accumulation toxicity. In all three cases, the same technique for passing the 18 G peridural catheter through a hole made with an 18 G intravenous catheter in the caudal space was used. Here, the initial dose of local anesthetic is administered, and, later, the peridural catheter is inserted to the required point. We use adult catheters in children given the difficulties involved in obtain pediatric kits in our context, the fact that we have more experience using this caliber of catheters, and that the benefits in postoperative care outweigh the risks. We have not needed to add opioids to the mix in any case since there was adequate pain management and opioids would represent a risk of respiratory depression.
Prolonged analgesia in very young patients poses a risk for the anaesthetist. In our setting, there is fear to use peridural catheters in neonates and infants. In the majority of cases, analgesia in these patients is limited to a single caudal dose with adequate analgesia for 6-8 h, requiring increased use of opioids during the postoperative period, with the risk of apnea, pruritus and urinary retention. Some pathologies require prolonged analgesic management as is the case in advanced malignant disease, pathologic fractures with nerve compression, severe trauma, and major abdominal or pelvic surgery.
The use of caudal catheters allows the epidural administration of local anaesthetic dilutions with adequate pain control, but the risk of infection is significantly increased because of proximity of the insertion site to the perineum. Caudal catheters become colonized after 48-72 h and, therefore, their use is not recommended beyond that time4. Tunnelling prolongs catheter dwelling time with better analgesic results, less infection and improved surgical outcome7.
Lower serum protein levels and metabolic immaturity of the neonate imply a higher risk of toxicity from local anaesthetics, related to a higher fraction of non-protein-bound anaesthetic. There is evidence of a continuous rise in plasma levels after 4S h of infusion4. The use of epidural lidocaine infusions has been shown to provide adequate long-term pain control; its shorter half-life allows for better management of plasma levels and results in a lower risk of toxicity when compared to bupivacaine. There are reports of successful epidural lidocaine infusions in neonates up to 30 days, with no signs of local anaesthetic toxicity at a dose of 0.S mg/kg/h (19.2mg/kg/day)4. In infants, the use of continuous bupiva-caine infusion is less risky because of their greater metabolic maturity and higher serum protein levels.
Local anaesthetic infusions are generally prepared with 0.1% bupivacaine, combined or not with opioids. In cases of analgesia below T10, a dose of 0.2 mg/kg/h (4.Smg/kg/day) is recommended; if analgesia above that level is required, the starting dose is 0.3 mg/kg/h (7.2 mg/kg/day) without exceeding the upper limit of 0.4mg/kg/h (9.6mg/kg/day)S. The continuous infusion of diluted lidocaine from 0.1% to 0.5% may be given in a dose of up to 1-1.5 mg/kg/h (24-36 mg/kg/day). However, considering the progressive increase in plasma levels of the anaesthetic, it is not recommended to maintain doses greater than 0.8 mg/kg/h (15.3 mg/kg/day) for more than 2 days. Local anaesthetic admixtures must be prepared every 72 h4,8. The concomitant use of intravenous multimodal analgesia with opioids, anti-inflammatories and benzodiazepines is indicated for maintaining adequate patient immobilization and sedation.
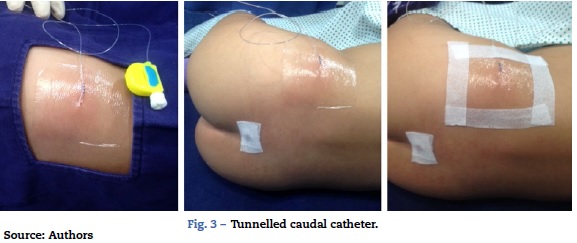
It is not easy to maintain tunnelled caudal catheters in place. In children, a high percentage of these catheters become displaced accidentally. In our institution, we avoid the use of stitches for securing the catheter by approaching the insertion point carefully and by covering the catheter exit point with transparent material so that signs of local infection can be detected early on. (Fig. 3).
Conclusion
Prolonged analgesia is essential for successful correction of bladder exstrophy. The use of tunnelling for caudal catheter insertion minimizes the complications associated with opioids and other sedative medications required for adequate patient immobilization, reducing the risk of apnoea and allowing adequate analgesia for more than 3 weeks with a lower risk of insertion site infection. It is important to have well-trained staff for catheter placement and for the follow-up of toxicity associated with local anaesthetics. Efficient communication between the anaesthesia team and the intensive care, orthopaedics and urology services is a pre-requisite for correct analgesic management of these patients.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflict of interest
The authors declare having no disclosures.
Funding
The authors did not receive sponsorship to undertake this article.
References
1. Wiersma R. Overview of bladder exstrophy: a third world perspective. J Pediatr Surg. 2008;43:1520-3. [ Links ]
2. Massanyi E, Gearhart J, Sabine K. Perioperative management of classic bladder exstrophy. Res Rep Urol. 2013;5:67-75. [ Links ]
3. Husmann DA. Surgery insight: advantages and pitfalls of techniques for the correction of bladder exstrophy. Nat Clin Pract Urol. 2006;3:95-100. [ Links ]
4. Kost-Byerly S, Jackson E, Yaster M, Kozlowski L, Mathews R, Gearhart J. Perioperative anesthetic and analgesic management of newborn bladder exstrophy repair. J Pediatr Urol. 2008;4:280-5. [ Links ]
5. Todd Purves J, Gearhart J. Pelvic osteotomy in the modern treatment of the exstrophy - epispadias complex. Eur Assoc Urol Update Ser. 2007;5:188-96. [ Links ]
6. Elsayed E, et al. Closure of bladder exstrophy with bilateral anterior pubic osteotomy: Revival of an old technique. Arab J Urol. 2011;9:203-7. [ Links ]
7. Laxmikant S, Borwankar S. Factors responsible for successful primary closure in bladder exstrophy. Pediatr Surg Int. 2000;16:194-8. [ Links ]
8. Aram L, Krane E, Kozloski L, Yaster M. Tunneled epidural catheters for prolonged analgesia in pediatric patients. Anesth Analg. 2001;92:1432-8. [ Links ]











 texto en
texto en