Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.3 Bogotá jul./set. 2013
Hepatopathology for gastroenterologists and hepatologists. Part Two
Useful terminology in the interpretation of the histopathological findings
Rocío del Pilar López Panqueva, MD. (1)
(1) Chief of Anatomical Pathology at the Fundación Santa Fé de Bogotá University Hospital. Professor in the School of Medicine at the Universidad de los Andes. Bogotá, Colombia. E-mail: rocio.lopez@fsfb.org.co - rolopez@uniandes.edu.co
Received: 24-06-13 Accepted: 26-06-13
Abstract
To understand the mechanisms of hepatic diseases it is indispensable to combine anatomical and histological concepts with physiopathological processes that occur in each illness. This first article in this series develops basic concepts of diagnostic histopathology.
Key words
Hepatic diseases, histology, hepatology, diagnostic histology, hepatic cells.
Histopathological studies of the liver must determine the existence not only of architectural abnormalities but also abnormalities each of the liver's cellular components. The purpose of this second article is to show the morphological changes that can occur in an injury and the terminology that describes each of these changes.
HEPATOCELLULAR CHANGES
Since hepatocellular changes occur in response to an injury of any etiology that disrupts cellular homeostasis, they can be observed in a large number of entities and can be the key for further diagnosis. Cellular injury can occur as a result of many causes including a hypoxic event, ischemia, infection, toxic drug injury, and genetic or immunological metabolic imbalances. Morphological expressions are equally variable and depend on the type of injury, and its severity and reversibility. We proceed to list the most important and frequently used expressions:
Cellular edema, ballooning change and ballooning degeneration
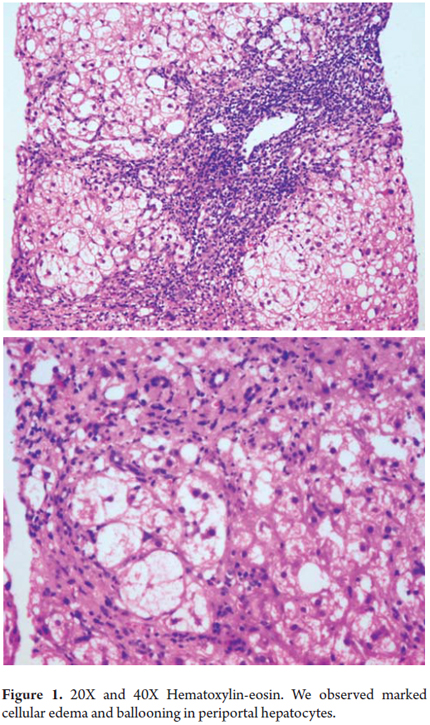
Increased cell volume can be both a physiological event with an important role in cell metabolism and an oxidative stress response to environmental or hormonal changes. It may also be a response to cellular injury by ATP depletion or impaired cell membrane disruption of the intermediate cytoplasmic filament mesh.
Morphologically, cells with large clear vacuolated cytoplasm that are 2 or 3 times the normal size of a hepatocyte are seen together with an accumulation of intermediate filaments resembling an amorphous pink material deposited in the cell cytoplasm. Ultra-structurally this can be seen as mitochondrial edema in cell membrane projections. This change can be seen isolated in cell groups or as a diffuse manifestation (Figure 1) (1).
Apoptosis, Acidophilic bodies or Councilman bodies
Apoptosis is a form of cellular death in which there is rupture of the cytoplasmic membrane with nuclear chromatin condensation and nuclear and cellular fragmentation. These changes occur as a result of the activation of caspases and endonucleases that induce alteration of the structural proteins and DNA. Apoptosis is a normal finding during liver development and remodeling, but it is also a frequently observed change in acute or chronic inflammatory processes such as viral hepatitis, autoimmune liver disease, steatohepatitis, and drug-toxic induced liver disease.
We observe hepatocytes that are greatly diminished in size with rhomboid or rounded forms with intensely eosinophilic cytoplasm and dark or pyknotic nuclei. When fragmentation occurs in the cytoplasm of these cells, acidophilic bodies form with complete loss of their nuclei (Figure 2) and escape into the sinusoidal space where they will subsequently be phagocytosed by Kupffer cells (2).
Focal necrosis or "spotty" necrosis and confluent necrosis
Focal necrosis is a form of cell death which differs from apoptosis. While cellular edema and focal necrosis both feature cellular edema, in focal necrosis vacuolization, karyolysis and release of cell contents also occur.
In focal necrosis, cell death occurs as the result of accumulation of lymphocytes and hypertrophic Kupffer cells containing liposfuscin, a brown material composed of lysate and phagocytosed detritus which is Periodic acid-Schiff (PAS) positive. At these necrosis sites there is no collapse and rupture of the reticular pattern.
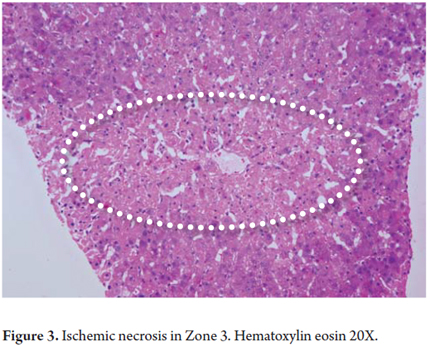
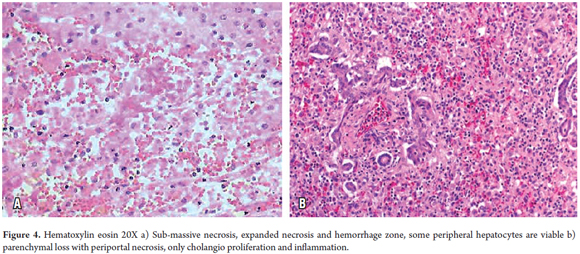
In confluent necrosis, cell death is distributed in an acinar or zonal pattern that can compromise large parenchymal areas with complete collapse of the reticular pattern. Its presentation depends on its severity. Centrilobular necrosis can be observed in an ischemic event resulting from hypovolemic shock or induced by drugs or toxicity (Figure 3). Portal-portal confluent necrosis and porta-central confluent necrosis are seen in chronic hepatitis while midzonal necrosis is seen during eclampsia. Large areas can be compromised in massive or sub-massive necrosis as observed in fulminant hepatitis (Figure 4). Zonal periportal necrosis is rare (3).
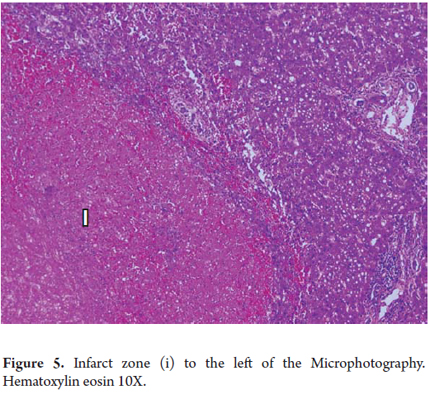
Coagulation necrosis or coagulation degeneration
Coagulation necrosis is observed in vascular injuries such as heart attacks, in yellow fever or hemorrhagic fever of viral etiology. It can also be seen in some toxic injuries such as acetaminophen poisoning, in tumoral necrosis and after procedures such as chemo-embolization, radiofrequency ablation, and ethanol injections for tumoral diseases such as hepato-carcinoma.
The eosinophils are very clear, have lost cellular detail, and appear to be shadow cells (Figure 5).
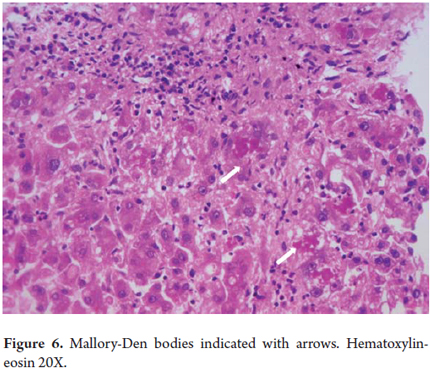
Mallory bodies, Mallory hyaline or Mallory-Denk Bodies
Mallory bodies are always ballooned liver cells containing clumps of irregular eosinophilic cytoplasmic within them (Figure 6). These differentiated hepatocytes have a characteristic pattern of expression of Type I (CK8) and Type II (CK18) keratins. They were initially described in 1911 by Dr. Frank D Mallory in relation to alcoholic hepatitis but have been observed in a number of other liver diseases including nonalcoholic steatohepatitis (NASH), Wilson's disease, chronic cholestatic diseases, toxic reactions to medications such as amiodarone, and hepatocellular carcinoma. They have recently been renamed Mallory Denk bodies (4, 5).
Oncocytic or oxyphilic alterations
Oncocytic and oxyphilic alterations have an intensely eosinophilic appearance with fine granules in the cytoplasm of the hepatocytes which indicates the presence of numerous mitochondria. Sometimes very large individual mitochondria, called megamitochondria, become prominent. These can be observed in alcoholic patients. They are PAS negative unlike α-1 antitrypsin globules which are PAS positive.
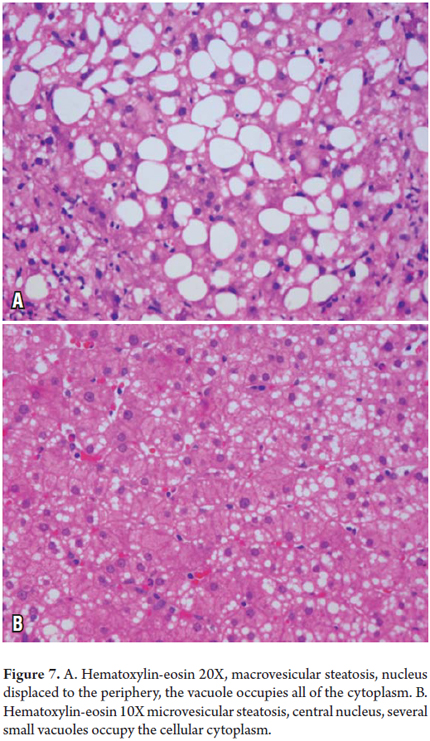
Fatty alteration or Steatosis
Steatosis is the accumulation of fat globules in the cytoplasm of hepatocytes which reflects an imbalance between the synthesis and secretion of lipids. It can be seen as a single morphological finding or it can be part of another type of liver injury.
In macrovesicular steatosis a large fat vacuole moves the nucleus towards the periphery. This occurs in both alcoholic and non-alcoholic fatty liver disease as well as in obesity, diabetes, diseases associated with abnormal lipid metabolism, hepatitis C, malnutrition, and drug toxicity.
In microvesicular steatosis we observe several small drops or intracytoplasmic fat vacuoles that displace the liver cell nucleus as in the acute fatty liver of pregnancy, Reye syndrome, valproic or tetracycline acid toxicity, and in alcoholic liver disease (Figure 7 A and B) (6).
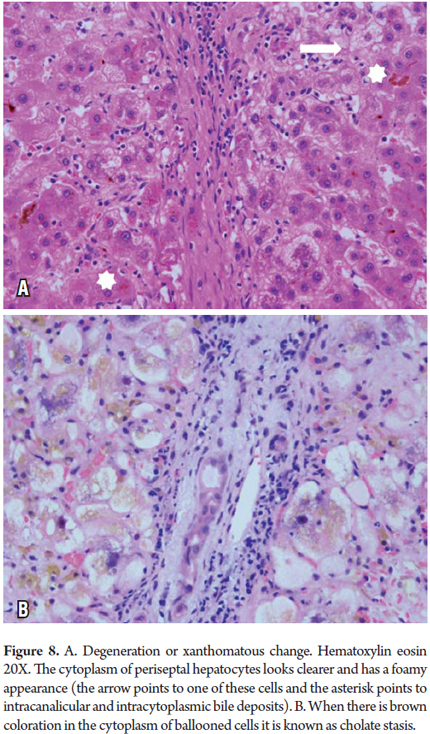
Xanthomatous degeneration, Cholate Stasis and Pseudoxanthomatous degeneration
These conditions are most frequently observed in chronic cholestatic diseases and obstructive cholestatic diseases. Accumulation of bile constituents which normally are secreted in the bile when the bile salts are toxic to the hepatocyte produces cellular edema with aggregates with brown pigmentation (Cholate stasis) in the cytoplasm which gives the periportally localized beginning stage hepatocytes a pale foamy appearance (Figure 11). It is not unusual to find Mallory-Denk bodies. As copper is a metal secreted by the bile it can also be demonstrated in these cells with special copper coloring or with a binding protein (Figure 8) (6, 7).
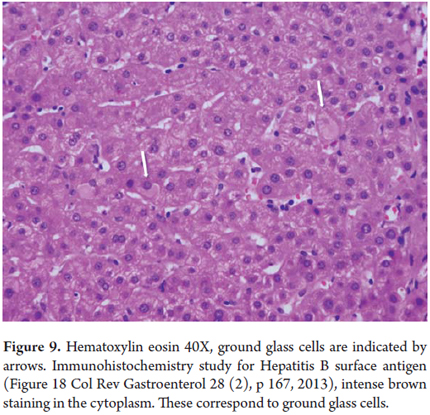
Ground glass cytoplasm
Ground glass cytoplasm has the appearance of a uniform very pale pink inclusion in the cell cytoplasm indicates proliferation of smooth endoplasmic reticulum (Figure 9). This has been observed in hepatitis B due to the accumulation of surface antigen (HBsAg) which is mixed with the endoplasmic reticulum. It tests positive when stained with orcein, aldehyde fuschine and Victoria blue and can be detected with immunohistochemical studies. It can also be a response to chronic ingestion of some drugs such as chlorpromazine, barbiturates, azathioprine, steroids, phenytoin, cyanamide, some analgesics and multiple immunosuppressive drugs after liver transplant. In addition to the clinical history, histochemical and immunohistochemical studies are useful for differential diagnosis (8,9).
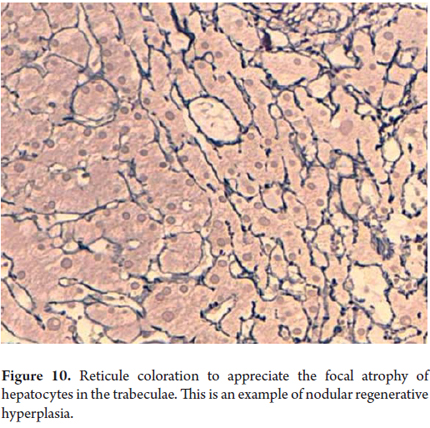
Cellular atrophy, trabecular atrophy
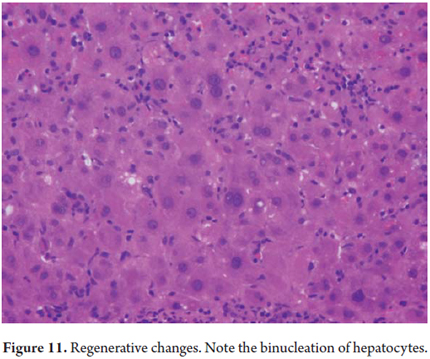
Cellular atrophy and trabecular atrophy consist of attenuation or flattening of hepatocytes with decreases in their size (Figure 10). Thinned trabeculae with reticular preservation can be observed. These conditions accompany vascular diseases such as regenerative and hyperplastic alterations. Regenerative changes are morphologically expressed as anisonucleosis with prominent nucleoli, binucleation and mitosis (Figure 11).
Cellular architecture can also change with double plaques and regenerative nodules. Giant cell transformation and multinucleated hepatocytes are the most commonly observed changes in neonatal hepatitis.
BILIARY SYSTEM CHANGES
Bile canaliculi
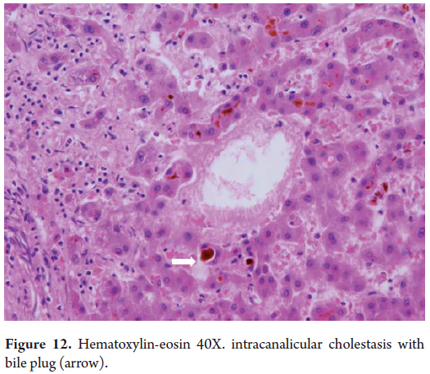
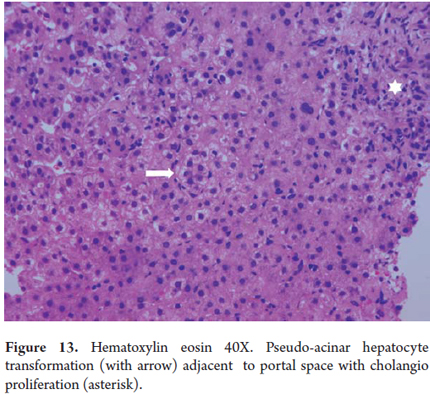
Bile canaliculi are only observed when they are dilated and in response to bile stasis. While the bile plugs are dilated canaliculi form by accumulation and bile pigment precipitation (Figure 12). Occasionally hepatocytes are arranged around the canaliculi giving themt a pseudo-glandular appearance (Figure 13).
Ductules, cholangioles and canals of Hering
These alterations are normally inconspicuous or invisible. Proliferation of cholangioles occurs in many diseases including in periportal areas near necrosis or scarred areas arranged in the periphery away from the arterial branches (Rev Col Gastroenterol 28 (2) pp. 162, 2013) (Figure 8).
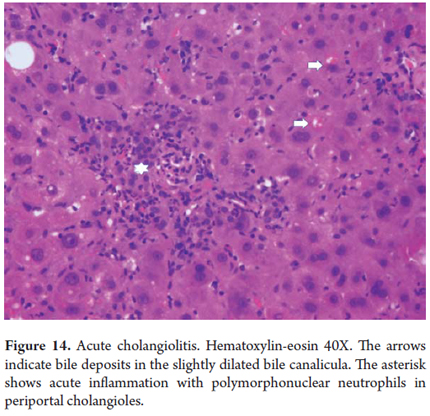
Acute cholangiolitis
Acute inflammation can accompany proliferation of cholangioles without having a special meaning by itself. It is different when it is associated with bile stasis with dilation of periportal cholangioles indicating a severe infectious process usually accompanied by sepsis (Figure 14).
Interlobular or acinar bile ducts
Ductal epithelium damage with degenerative vacuolization, nuclear pseudo-stratification, intraepithelial inflammatory cell permeation and/or ductal destruction are indications for these conditions. They accompany many biliary tract diseases and acute cellular rejection phenomena in post-transplant patients.
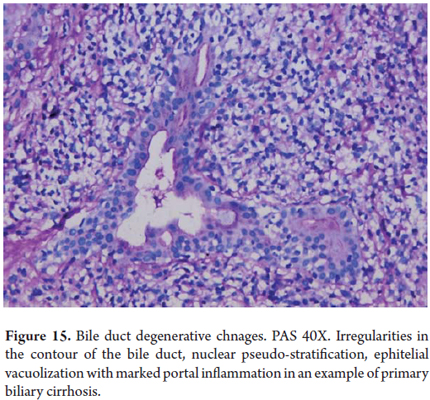
Acute inflammation or acute cholangitis may indicate mechanical obstruction, whereas inflammation or nonsuppurative cholangitis are accompanied by degenerative changes which are most frequently observed in chronic cholestatic diseases such as primary biliary cirrhosis (Figure 15) and primary sclerosing cholangitis.
Formation of dilated bile ducts with bile infarcts is typical of large duct obstructions.
VASCULAR CHANGES
When we refer to hepatic vascular structures, we are talking of large vessels and the branches of the portal vein, the hepatic artery, the terminal and central venules and sinusoids. We can observe vascular lesions primarily or secondarily to several systemic entities, and there are acute forms, chronic forms, and hereditary and congenital lesions. Classification requires adequate clinical information correlated with the observed morphological changes (10).
Thrombotic occlusion causes ischemic necrosis or sinusoidal congestion changes. Portal or hepatic vein thrombosis (Budd-Chiari syndrome) can have multiple causes such as hypercoagulable states, presence of masses, inflammatory, infectious or congenital vascular lesions, and surgical manipulation.
Inflammation produces phlebitis or arthritis.
Microvasculature compromise, sinusoidal injury and veno-occlusive diseases have many morphological patterns according to their etiology, e.g. toxic or drugs, ischemic change, congestion and sinusoidal dilatation, inflammation or infiltration by amyloid or tumarl lesion or light chains.
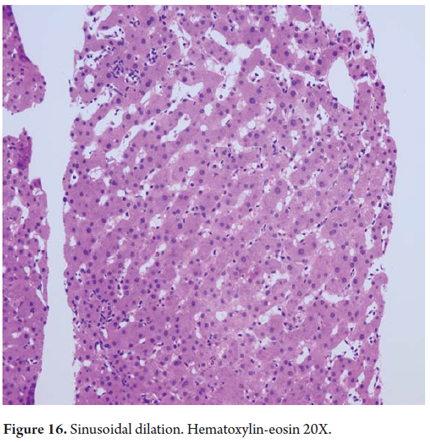
Zone 3 sinusoidal dilatation indicates venous outflow obstruction of any etiology (Figure 16) and while zone 1 sinusoidal dilatation can be caused by oral contraceptives.
Kupffer cells become very hypertrophied in response to physiological or pathological phagocytosis.
INFLAMMATORY CHANGES
We observe the intensity, location and cell type of inflammatory changes which may be the key to diagnosis of various entities (6).
Portal and periportal locations are found in chronic hepatitis and chronic cholestatic diseases. When there is acinar inflammation it is most often associated with acute viral hepatitis, toxic drug induced hepatitis and metabolic injuries. Peri-ductal and intraductal locations suggest biliary or vascular diseases.
Plasma cells and lymphocytes accompany autoimmune diseases while polymorphonuclear neutrophils are seen in acute bacterial infections, ascending cholangitis, and in areas of infarction. Eosinophils may suggest the possibility of drug-induced injury and is an important cellular element in acute cellular rejection after transplantation. Epithelial granulomas, with or without necrosis and with or without giant cells, have a very long list of possible differential diagnoses which include infectious diseases, drug toxicity, sarcoidosis, foreign body reaction, and autoimmune diseases.
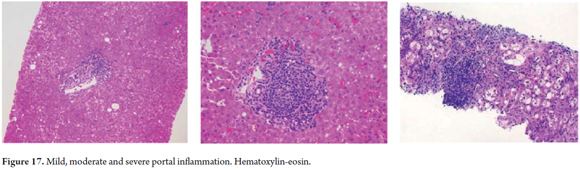
Knowing the amount and distribution of inflammatory cells is useful for grading the severity of liver compromise (Figure 17).
FIBROSIS AND CIRRHOSIS
A key indicator for pathological diagnosis of fibrosis and cirrhosis is in inflammation. Its location can vary from portal to periportal, periductal, intracinar or subsinusoidal. The distribution of the inflammation can be focal, diffuse, segmental or lobular. It can vary from severe to mild. Severe fibrosis with incomplete or complete cirrhotic nodules is the other key indicator.
The WHO classifies cirrhosis based solely on the size of the nodules: micronodular means less than 3 mm across, macronodular means larger than 3 mm in diameter, and mixed means a mixture of both (6).
Sometimes diagnosis of cirrhosis or fibrosis from a biopsy can be very difficult or impossible. Examples of difficult to diagnose biopsies include large cirrhotic nodules in highly fragmented samples partially surrounded by fibrous tissue, subcapsular biopsies, and biopsies taken from scar tissue.
Histochemical studies, most commonly Masson's trichrome with or without reticle eyepiece, are essential for assessment of fibrosis.
LESION PATTERNS
The combination of all of these changes gives us liver damage patterns characteristic of many entities. Recognition of them helps us establish and limit differential diagnosis from a biopsy or liver tissue study. We will list some of the patterns most frequently encountered in clinical practice, and we will describe them later in this series.
Necroinflammatory pattern: acute and chronic. Inflammation and cell death (apoptosis and/or necrosis), characteristic of viral autoimmune and some toxic-drug induced hepatitis.
Steatohepatitis pattern: characterized by the presence of steatosis in zone 3, inflammation and cell damage from ballooning, necrosis and/or pericellular or subsinusoidal fibrosis.
Cholestatic pattern: acute and chronic. Biliary stasis and swelling in acute cholestasis, bile deposits, xanthomatous degeneration, copper deposits, Mallory-Denk bodies in chronic cholestasis.
Vascular pattern: hepatocyte trabeculae atrophy, sinusoidal dilatation and congestion or infarcts.
REFERENCES
1. Farber JL, Kyle ME, Coleman JB. Mechanisms of cell injury by activated oxygen species. Lab Invest 1990; 62: 670-679. [ Links ]
2. Bai J, Odin JA. Apoptosis and the liver: relation to autoimmunity and related conditions. Autoimmunity Rev 2003; 2: 36-42. [ Links ]
3. Jaeschke H, Gujral JS, Bajt ML. Apoptosis and necrosis in liver disease. Liver Int 2004; 24: 85- 89. [ Links ]
4. Zatloukal K, French SW, Stumptner C, et al. From Mallory to Mallory-Denk bodies. What, How, and why? Exp Cell Res 2007; 313: 2033-2049. [ Links ]
5. French SW, Bardag-Gorce F, Li J et al. Mallory-Denk body pathogenesis revisited. World J Hepatol 2010; 2: 295-301. [ Links ]
6. Burt A, Portmann B, Ferrel L. MacSween's Pathology of the Liver. Sixth edition. Churchill Livingstone Elsevier 2012. [ Links ]
7. Zollner G, Trauner M. Mechanisms of cholestasis. Clin Liver Dis 2008; 12: 1-26. [ Links ]
8. Vasquez JJ. Ground-glass hepatocytes: light and electron microscopy. Characterization of the different types. Histol Histopathol 1990; 5: 379-386. [ Links ]
9. Lefkowitch JH, Lobritto SJ, Brown Jr RS, et al. Ground-glass polyglucosan-like hepatocellular inclusions: A new diagnostic entity. Gastroenterology 2006; 131: 713-718. [ Links ]
10. Deleve, Valla and Garcia-Tsao. Vascular disorders of the liver. Hepatology 2009; 5: 1729-1764. [ Links ]











 texto en
texto en