Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957versión On-line ISSN 2500-7440
Rev Col Gastroenterol v.24 n.3 Bogotá jul./sep. 2009
Hepatitis B infections in oncology patients. A survey of clinical practice
Marcela Urrego MD. (1), Aurelio Angulo MD. (2), Jaime Holguín MD. (3).
(1) Hematologist, Oncologist, MD at Imbanaco de Cali medical center, 2008 President of the Cali Cancer Congress. Cali, Colombia.
(2) Radiotherapist, MD at Valle de Lili Cali Foundation. 2008 Secretary of the Cali Cancer Congress. Cali, Colombia.
(3) Internist, Hepatologist, MD. Professor of Medicine at the Universidad del Valle. Cali. Colombia.
Received: 23-03-09 Accepted: 12-08-09
Summary
The risk of reactivating chronic hepatitis B during chemotherapy is being increasingly recognized. The aims of this study were to evaluate the degree of awareness among hematologists and oncologists in Colombia regarding this potential risk, and to examine screening and prevention practices.
Methods: A written questionnaire was used to survey physicians attending the National Congress of Cancer held in Cali in October 2008.
Results: 134 physicians who specialize in treatment of oncology patients were surveyed.
Over half (58 %) have more than 10 years in clinical practice; 23 % never screened patients for hepatitis B infection prior to initiating chemotherapy, 50 % screened patients for hepatitis B " sometimes ", while only 27 % screened all patients before chemotherapy. Only 23 % selected the serological tests specifically for hepatitis B for patients needing chemotherapy. Most of these specialists prescribed pegylated interferon as their preferred prophylactic agent.
Conclusions: The oncology community needs to become more aware of the risks of hepatitis B reactivation during chemotherapy.
Key Words
Hepatitis B, reactivation, chemotherapy, oncologists
INTRODUCTION
It is estimated that 350 million people in the world currently suffer from chronic Hepatitis B virus (HBV) infections. 30% of those infected develop cirrhosis, hepatic insufficiency and/or hepatocellular carcinoma. HBsAg prevalence varies among countries: high prevalence areas have HBsAg > 8 %; intermediate areas have HBsAg between 3% and 7 %; while low prevalence areas have HBsAg < 3 %.
Although Colombia is considered to be a low prevalence country, high prevalence zones have been identified on the Pacific coast, in the Sierra Nevada de Santa Marta and in Amazon jungle areas near the borders with Brazil and Venezuela. Patients that develop chronic infections generally appear to be indolent, sometimes develop symptoms such as arthralgias or fatigue, and only in very advanced stages show recognizable symptoms associated with chronic hepatic illness.
It is well known that exposure to immunosuppressive therapy, chemotherapy or cytotoxic agents can reactivate inactive HBV infections (1). The natural courses of these outbreaks vary. The vast majority of cases develop asymptomatically and are accompanied by aminotranspherases (ALT) and viral DNA elevation. However, cases of patients who have developed jaundice, hepatic decompensation and acute liver failure, as well as patients who have died, have also been described in the literature.
The incidence of Hepatitis B reactivation among chemotherapy patients ranges between 10% and 50%, with mortality rates ranging from 4% to 60%. HBV reactivation is more frequent when protocol corticosteroids and anthracyclines are included during chemotherapy. On the other hand, between 37% and 57% of post- kidney transplant patients die of liver disease progression. Recently, reactivation and symptoms of severe hepatitis have been described in cases in which patients were treated with immunosuppressants and immunomodulatory agents including rituximab, infliximab and other anti-TNF used for rheumatic diseases or inflammatory bowel disease.
In 2007, the American Association for the Study of Liver Diseases (AASLD) published guides for the treatment of HBV infected patients which recommended screening for HBsAg in all patients about to begin therapy with cytotoxic or immunosuppressive agents. The aim is to identify patients at risk of HBV infection and HBV reactivation so that appropriate health measures can be taken in a timely manner.
Evidence showing low screening frequency supports these recommendations. In October, 2007 Tram Tran and collaborators (3) published the results of a survey in the United States which found that 20% of oncologists never tested for HBV prior to start of chemotherapy, while only 38% screened their patients for HBV infection risk.
In Colombia information on this topic is limited to anecdotal reports of decompensation and acute liver failure associated with the use of immunosuppressive and anti-TNF agents. Normal oncological practice was not known.
The objective of the present study was to evaluate Colombian oncologists level of knowledge about the risk of HBV reactivation in their patients, as well as to learn about normal examination and prevention practices.
MATERIALS AND METHODS
A survey designed to answer these questions was distributed to oncologists and hematologists attending the 2008 Colombia Cancer Congress held in Cali in October, 2008.
Doctors attending the Congress voluntarily answered 7 multiple choice questions. The surveyors, a clinical secretary, a graduate nurse and an assistant, did not influence the responses of physicians surveyed. See appendix 1.
The survey questions asked about years of experience in clinical practice, type of Hepatitis B serological tests used, frequency of test use prior to starting chemotherapy, and screening for risk factors related to Hepatitis B infection. The survey also inquired about preferred treatment options for patients diagnosed with Hepatitis B virus.
RESULTS
A total of 290 doctors voluntary answered the survey: 60 oncologists (21%); 31 hematological oncologists (11%); 29 urologists (10%); 26 radiotherapists (9%); 20 oncological surgeons (7%); 17 hematologists (6%); 17 gynecologists (6%); 6 pediatric oncologists (2%); 5 neurosurgeons (2%); 5 otolaryngologists (2%); 3 pediatricians (1%), and 71 physicians with other specialties (25%) (internists, epidemiologists, surgeons, and orthopedists).
Of the total surveyed, 134 are specialists who prescribe chemotherapy in Colombia. They include the Oncologists (60), hematological oncologists (31), hematologists (17), pediatric oncologists (6) cancer surgeons (20).
CLINICAL EXPERIENCE
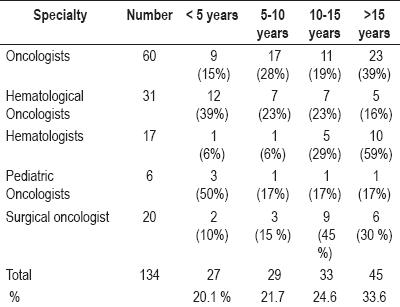
27 of the 134 doctors who prescribe chemotherapy (20.1%) have less than 5 years of experience. 15% of the oncologists and 39% of the hematological oncologists have less than 5 years of experience.
In contrast, 59% of hematologists have over 15 years of experience, and 75% of surgical oncologists have more than 10 years of work experience (table 1).
Table 1. Clinical experience.
FREQUENCY OF USE OF SCREENING TESTS FOR HBV
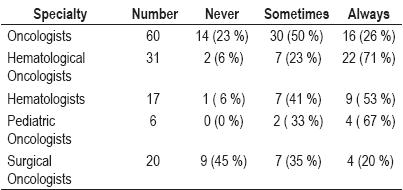
23% of the oncologists do not test for hepatitis B. 50% sometimes do while only 26% always test for Hepatitis B prior to chemotherapy.
71% of the hematological oncologists and 53% of hematologists always test for hepatitis B prior to chemotherapy.
Pediatric oncologists test for Hepatitis B prior to chemotherapy most frequently: 33% sometimes test, while 67% always test.
45% of surgical oncologists do not test for Hepatitis B (table 2).
Table 2. Screening frequency.
SCREENING TESTS USED BY PRACTITIONERS
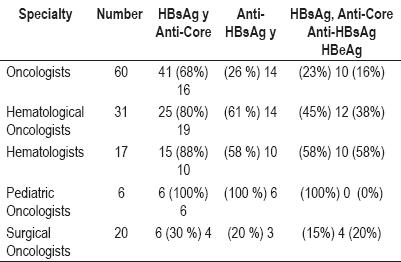
68% of the oncologists order HBsAg and anti-Core testing, while 26% order anti-HbsAg testing. All three tests (HBsAg, Anti-Core, Anti-HBsAg) are ordered by 23% of oncologists.
80% of the hematological Oncologists order HBsAg and anti-Core testing while 61% order anti-HbsAg. All three tests are requested by 45%.
88% of hematologists request HBsAg and anti-Core testing while 58% order Anti-HbsAg testing. 58% request all three tests.
All pediatric oncologists ask for the three tests: HBsAg, Anti-HBsAg and anti-Core.
30% of surgical oncologists request HBsAg and anti-Core testing, 20% request anti-HbsAg testing, while all three tests are requested by just 15% (table 3).
Table 3. Screening tests used.
FREQUENCY OF SCREENING FOR HEPATITIS B RISK FACTORS
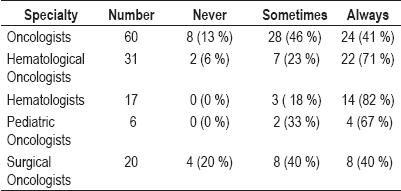
41% of the Oncologists always look for Hepatitis B risk factors in their patients. 71% of the Hematological Oncologists always test for Hepatitis B risk factors, while 82% of the Hematologists always look for Hepatitis B risk factors. Among the Pediatric Oncologists 67% always look for Hepatitis B risk factors among their patients, while 40% of the surgical oncologists do (table 4).
Table 4. Frequency of screening for hepatitis B risk factors.
Hepatitis B treatments
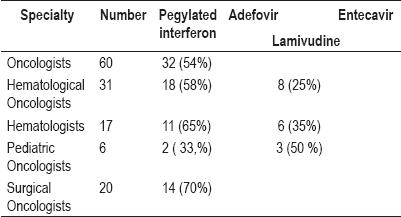
Most of these specialists prefer pegylated interferon for treatment of these patients: 54% of the Oncologists preferred pegylated interferon (the remainder,46%, did not answer this question); 58% of the Hematological Oncologists selected pegylated interferon; and 70% of surgical oncologists preferred pegylated interferon. However, 50% of Pediatric Oncologists select lamivudine, while this drug was chosen by 35% of the hematologists and 25% of the Hematological Oncologists.
No doctors chose adefovir or entecavir as a treatment option for hepatitis B patients (table 5).
Table 5. Treatments chosen for hepatitis B.
DISCUSSION
A very significant group of Colombian specialists who treat cancer patients was surveyed: 60 Oncologists, 31 Hematological Oncologists, 17 Hematologists, 6 Pediatric Oncologists and 20 Surgical Oncologists. More than half, (58.2%) of these specialists have over 10 years of experience in their specialty.
This study highlights the facts that 23% of oncologists and 45% of surgical oncologists do not request testing for Hepatitis B prior to initiation of chemotherapy, while 41% of hematologists and 50% of oncologists only sometimes " order tests for Hepatitis B before chemotherapy.
All of the Pediatric oncologists surveyed selected all three hepatitis B tests that should be requested prior to initiation of chemotherapy: HbsAg, Anti-Core, Anti-HBsAg). However only a small percentage of other specialists chose all three tests: 23% of Oncologists; 15% of the Surgical Oncologists and 45% of the Hematological Oncologists. Most practitioners in these specialties exhibited flawed judgment in the selection of appropriate tests.
That screening for Hepatitis B risk factors in oncology patient is not a practice well established practice is evidenced by the facts that 13% of Oncologists never look for these risk factors, while 46% only look "sometimes". Hematologists were the group that most often looks for this risk (82%).
When selecting the appropriate drug for hepatitis B prophylaxis in oncology patients, the vast majority of experts prefer pegylated interferon contrary to most international guidelines that recommend the use of lamivudine. Strikingly, there were no specialists who considered adefovir or entecavir treatments as options for patients.
This result suggests that in the actual clinical practice of specialists treating hemato-oncological cases, HBV screening is not a well established practice even though international guidelines appeared in 2007. Also, the use of lamivudine as a prophylactic antiviral therapy for oncology patients with HBV infections does not seem to be a common practice.
These data support the initiative to conduct more academic forums in which experts in infectious diseases and hepatology can discuss with doctors specializing in oncology patient management. International guidelines have been issued which could generate recommendations for all oncology patient care centers in Colombia.
Beginning in 1975 American and British reports began describing Hepatitis B reactivation with exacerbation of liver disease and recurrence of HBsAg (4, 5) in patients treated with chemotherapy for lymphoma or leukemia. Since then many hepatitis B reactivation reports have been published. Reactivation can occur with chemotherapy for solid tumors and for leukemia; with rituximab use; with immunomodulatory agents such as prednisolone or infliximab for fundamental autoimmune diseases; with progression of HIV; after solid organ transplants (kidney, heart, lung and liver) and most dramatically and commonly, after bone marrow transplantation (6, 7).
Several controlled clinical studies have shown the efficacy of nucleoside analogues (particularly lamivudine) in reducing Hepatitis B reactivation, clinical hepatitis, and death associated with hepatitis B viral injury (8).
There are three clinical situations in which Hepatitis B reactivation can occur.
In patients with chronic liver disease, with detectable DNA, the higher the viremia level, the greater is the risk of exacerbation. Immunosuppression in these patients leads to increased levels of DNA and decreased levels of ALT. When immunosuppression is suspended, reconstitution of the immune system leads to aggressive and often fatal liver damage. It is recommended that patients with active liver disease and elevated levels of DNA should be treated with antiviral therapy before, during and after immunosuppression. Potent analogues with high genetic barriers such as Entecavir and Tenofovir are the most recommended (9). Lamivudine prophylaxis is insufficient for these patients.
Inactive HBsAg carriers may suffer reactivation directly related to the duration and potency of immunosuppressive agents or chemotherapy. Reactivation generally occurs when therapy is suspended or between cycles of chemotherapy. In these patients lamivudine or adefovir treatment should start 3-4 weeks before chemotherapy and should continue for 2 to 6 months after completion of chemotherapy.
Patients with reactive anti-core, with anti-HBsAg + / -, with negative HBsAg and undetectable DNA in serum may be Hepatitis B virus carriers with DNA detectable only in liver tissue. When subjected to prolonged or deep immunosuppression such as following solid-organ transplantation or after bone marrow transplantation, these patients can develop a severe clinical condition called "reactivation with reappearance of HBsAg," which can progress to liver failure and which can be fatal. The American society for the Study of Liver diseases (ASSLD) recommends that these patients should receive nucleoside analogues throughout the period of chemotherapy and continue for 6 months after the end of chemotherapy (10).
Acknowledgments
This work was possible thanks to the valuable collaboration of the following:
The board of Colombian Cancer Association which organized the 2008 Colombian Cancer Congress that took place in the city of Cali from October 30 to November 2, 2008.
Dr. Maria del Rosario Olivera, specialist doctor in clinical epidemiology who reviewed and corrected our work.
Ana Milena Rodriguez Ocampo, a nurse, Elizabeth Bonilla, a secretary and our assistant Claudia Marcela Florez for their support in implementing the survey.
Viral diseases in oncology patients
Survey approved by 2008 Cancer Congress board.
REFERENCES
1. Yeo W, Chan PKS, Zhong S, et al. Frecuency of hepatitis B reactivation in cancer patients undergonig cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol 2000; 62: 299-307.
2. Lam KC, Lai CL, Trepo C, Wu PC. Deleterious effect of prednisolone in HBsAg positive chronic active hepatitis. N Eng J Med 1981; 304: 380-386.
3. Tram T, Mina O, Poordad F, Martin P, Screening for hepatitis B in chemotherapy patients: Survey of current oncology practice. Hepatology 2007; ASLD abstracts # 988.
4. Galbraith RM, Eddleston AL, William R, et al. Fulminant hepatic failure in leukemia and choriocarcinoma related to withdrawal of cytotoxic drug therapy. Lancet 1975; 2: 528-530.
5. Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myelo-proliferative and lymphoproliferative disordes. Gastroenterolgy 1975; 68: 105-112
6. Waite J, Gilson RJ, Weller IV, et al. Hepatitis B virus reactivation or reinfection associated with HIV infection. AIDS 1988; 2: 443-448.
7. Knoll A, Boehm S, Hanh J, Holler E, Jilg W. Reactivation of resolved hepatitis B virus infection after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 2004; 33: 925-929.
8. Loomba R, Rowley A, Wesley R, et al. Systematic review: The effect of preventive Lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 2008; 148: 519-528.
9. Lok AS, McMahon BJ, Chronic hepatitis B.AASLD practice guidelines. Hepatology 2007; 45: 507-539.
10. Liaw YF, Leung N, Guan R, et al. Asian- pacific consensus statement on the management of chronic hepatitis B: a 2005 Update. Liver Int 2005; 25: 472-480.
1. Yeo W, Chan PKS, Zhong S, et al. Frecuency of hepatitis B reactivation in cancer patients undergonig cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol 2000; 62: 299-307. [ Links ]
2. Lam KC, Lai CL, Trepo C, Wu PC. Deleterious effect of prednisolone in HBsAg positive chronic active hepatitis. N Eng J Med 1981; 304: 380-386. [ Links ]
3. Tram T, Mina O, Poordad F, Martin P, Screening for hepatitis B in chemotherapy patients: Survey of current oncology practice. Hepatology 2007; ASLD abstracts # 988. [ Links ]
4. Galbraith RM, Eddleston AL, William R, et al. Fulminant hepatic failure in leukemia and choriocarcinoma related to withdrawal of cytotoxic drug therapy. Lancet 1975; 2: 528-530. [ Links ]
5. Wands JR, Chura CM, Roll FJ, Maddrey WC. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myelo-proliferative and lymphoproliferative disordes. Gastroenterolgy 1975; 68: 105-112 [ Links ]
6. Waite J, Gilson RJ, Weller IV, et al. Hepatitis B virus reactivation or reinfection associated with HIV infection. AIDS 1988; 2: 443-448. [ Links ]
7. Knoll A, Boehm S, Hanh J, Holler E, Jilg W. Reactivation of resolved hepatitis B virus infection after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 2004; 33: 925-929. [ Links ]
8. Loomba R, Rowley A, Wesley R, et al. Systematic review: The effect of preventive Lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 2008; 148: 519-528. [ Links ]
9. Lok AS, McMahon BJ, Chronic hepatitis B.AASLD practice guidelines. Hepatology 2007; 45: 507-539. [ Links ]
10. Liaw YF, Leung N, Guan R, et al. Asian- pacific consensus statement on the management of chronic hepatitis B: a 2005 Update. Liver Int 2005; 25: 472-480. [ Links ]











 texto en
texto en