Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.3 Bogotá set. 2014
Prevalence of food allergies in patients with cystic fibrosis seen in the Pediatric Gastroenterology, Hepatology and Nutrition Service of Gastronutriped in Bogotá between 2009 and 2013
Wilson Daza MD. MSc. (1), Silvana Dadán MD. MSc. (2), Ana María Rojas MD. (3)
(1) Pediatric Gastroenterologist, Masters Degree in Clinical Nutrition, Associate Professor of Pediatrics and Pediatric Gastroenterology at the Universidad El Bosque, Director of Gastronutriped IPS in Bogotá, Colombia. E-mail: wilson.daza@gastronutriped.com, dazawilson@unbosque.edu.co
(2) Clinical Nutritionist, Masters Degree in Clinical Nutrition, Associate Professor and Director of Graduate Pediatric Gastroenterology at the Universidad El Bosque, Clinical Nutrition Coordinator of Gastronutriped IPS in Bogotá, Colombia.
(3) Pediatrician, Pediatric Gastroenterology Fellow at the Universidad El Bosque in Bogotá, Colombia.
Received: 20-12-13 Accepted: 21-07-14
Abstract
Introduction: Food allergies and cystic fibrosis (CF) are complex diseases that may occur concomitantly. Although this seems uncommon, several cases of simultaneous presentation have been observed in our clinical practice. Given this, we developed an interest in observing the behavior of food allergies in CF patients seen at a referral clinic for both entities.
Objective: The objective of this study was to determine the prevalence of food allergies in cystic fibrosis patients at the outpatient pediatric gastroenterology clinic of Gastronutriped IPS in Bogotá between 2009 and 2013.
Methodology: This is a retrospective study of male and female patients diagnosed with CF and treated at the Unit of Gastroenterology, Hepatology and Nutrition of Gastronutriped IPS between 2009 and 2013. Patients ages ranged from newborns to 18 years old. We reviewed their medical records to determine whether they also had been diagnosed with any type of food allergy.
Results: We found that 14.8% (n = 4/27) of the CF population analyzed presented diagnoses of food allergy, although the clinical expressions of these allergies varied.
Conclusion: When gastrointestinal symptoms persist in patients with CF despite adherence to proper treatment, it is essential to suspect other independent pathologies that may compromise the gastrointestinal tract.
Keywords
Cystic fibrosis, food allergy, malabsorption syndrome, eosinophilic gastrointestinal disorders.
INTRODUCTION
Cystic fibrosis (CF) is a recessive autosomal entity conditioned by the mutation of a gene located in the long arm of chromosome 7 which encodes for the cystic fibrosis transmembrane conductance regulator (CFTR) and acts as a chlorine channel. When it undergoes modification, the transport of ions in the epithelium is altered including the epithelium of air passages and the pancreas (1-6). More than 1,800 mutations of the CFTR gene have been detected, but Delta F508 is the most common. It is found in 70% of CF patients (2, 8). Altered transport of ions through the apical surface of the epithelial cells is generated as a secondary effect of this mutation. This prompts the thickening of epithelial secretions resulting in a viscous surface. Similarly, it results in reduction of the action of mucociliary transport and beta-defensins which are local defense mechanisms and in increased pro-inflammatory activity (8).
Cystic fibrosis compromises the health of about 30,000 children and adults in the United States and around 80,000 individuals worldwide. In Colombia, exact figures are not available as data are under-recorded. In the year 2011, Vasquez documented that the incidence of cystic fibrosis in Latin-America is about 1 in 5,000 to 1 in 8,000 live births out of which about 500 patients were diagnosed in Colombia (9).
CF may express itself in the classical manner or in an atypical way depending in part on the mutation responsible for the ailment. The typical form involves the lungs and includes malnutrition, malabsorption syndrome with steatorrhea and is linked to meconium ileus. The atypical form leads to manifestations such as pancreatitis and bronchopulmonary disease, but with pancreatic sufficiency. Sweat electrolytes may or may not be altered sometimes resulting in late diagnosis. Inflammation of the paranasal sinuses and the nasal mucosa has been found in 74% to100% of patients, and nasal polyps have been found in in 6% to 44% (8).
Patients suffering from CF may present non-specific gastrointestinal symptoms such as intolerance to certain foods, failure-to-thrive, abdominal pain, and nausea and vomiting. These are generally associated with CF co-morbidities such as malabsorption, distal intestinal obstruction syndrome (DIOS), gastroesophageal reflux disease (GERD), and bacterial overgrowth (7). Nevertheless, in some cases gastrointestinal symptoms might lead to the suspicion of food allergies for which differential diagnosis should be considered although it is feasible that the patient suffers from both ailments. Nevertheless, in the scientific literature there have been few reports of this (7).
Food allergies have a wide variety of symptoms. They may compromise different organs such as the skin which manifests as itching, erythema, hives, angioedema, dermatitis; the gastrointestinal tract which manifests as nausea, vomiting, dysphagia, diarrhea, abdominal pain, colic, constipation, and rejection of food; or the air passages which manifests primarily as rhinoconjunctivitis and asthma. They can affect nutritional status through malnutrition and failure to thrive, and they may even induce anaphylaxis with serious high risk to the patient´s life (15).
In other words, food allergies presents a broad spectrum of gastrointestinal and nongastrointestinal manifestations. They can be placed into three groups in accordance with the triggering immune mechanisms:
1. Manifestations mediated by immunoglobulin E (IgE) which have acute onsets and compromise more than one organ including the skin (hives and angioedema), the respiratory system (rhinoconjunctivitis and asthma), and the gastrointestinal tract (nausea, vomiting and diarrhea).
2. Non-IgE-mediated reactions which have late onsets and chronic evolutions. These reactions include enterocolitis and proctocolitis.
3. Mixed IgE and non-IgE-mediated reactions which have late onsets and which may manifest as atopic dermatitis or as eosinophilic gastroenteropathies (4).
The incidence of food allergies is still uncertain; a prevalence of 52 cases/100,000 people is estimated in the United States, with similar numbers in Europe. A review of the scientific literature indicates that food allergies affect between 1% and 10% of the population, and that 8% of children have the disease: 2.4% present allergies to a wide range of foods, and 3% experience severe reactions. In addition, it has been observed that prevalence is increasing over time (10). One of the difficulties in calculating the incidence of food allergies is that the entity cannot always be clearly differentiated from other disorders such as the hypereosinophilic syndrome, connective tissue ailments, celiac disease, Crohns disease, parasitic infections, and drug hypersensitivity (7, 12, 13). Other factors that affect the calculation of prevalence include definitions of allergy, populations studied, methodology employed, geographic variations, ages and food exposures (10, 14, 15).
The most common allergens affecting pediatric patients include cows milk (2.2%), peanuts (1.8%), nuts (1.7%), soy, eggs, wheat, fish and sea food (10). Nevertheless, depending on the individual, practically any kind of food can have the potential to sensitize. In general, when a child initially presents an allergy to milk, eggs, wheat or soy, the allergy or allergies are very likely to resolve while allergies to peanuts, nuts, fish and sea food tend to persist (10).
Risk factors that influence sensitization and/or development of food allergies itself are male gender, ethnic group (greater risks for Asian children and African children than for white (Caucasian) children), genetics (family association, HLA and specific genes), atopy (comorbidity with atopic dermatitis), vitamin D deficiency, fat intake profile (low consumption of omega-3 polyunsaturated fatty acids), low consumption of antioxidants, use of antacids (due to reduced digestion of allergens), obesity (basic pro-inflammatory state), and extreme hygiene practices. Timing and route of exposure to food allergens are also factors because when the entry of allergens is delayed, the risk of developing an allergic reaction is increased.
Atopic diseases related to food allergies include asthma, atopic dermatitis, allergic rhinitis and sinusitis. Radioallergosorbent tests (RAST), assays with marked enzymes and skin tests are the most common diagnostic tests. When required, upper and lower endoscopy may also be used (7).
Treatment should be individualized according to the type of food allergies documented and diagnosed for each patient. For example, eosinophilic esophagitis should be managed pharmacologically together with an elimination diet designed according to the severity of the patients condition, availability of medication and the physicians experience. An elimination diet is easier and more effective if the responsible allergen has been detected. When the allergen has not been identified with ImmunoCAP from blood and/or skin tests, the most frequent allergens such as milk, eggs, soy, wheat, peanuts-nuts, fish, and sea food should be progressively restricted depending on medical criteria (7).
Since the symptoms of food allergies can be quite non-specific, and since food allergies may have symptoms in common with symptoms of CF, a diagnosis of food allergies may not be made. For patients suffering from CF who reject feeding, fail to thrive, or have persistent symptoms that cause emesis, regurgitation, heartburn or dysphagia despite treatment and adherence, the possibility of food allergies such as eosinophilic esophagitis should be considered. On occasion, an endoscopic procedure with a pathological study is required in order to confirm or rule out such a diagnosis (7, 12, 13).
Patients suffering from CF who are diagnosed with food allergies may require medication in addition to the basic CF therapeutic regime. This can become complicated. For example, if corticoids are needed they can negatively impact the adrenal axis and bone health which are usually already affected by CF (11).
In general terms, patients with food allergies and CF have even greater nutritional risks which makes a multidisciplinary monitoring approach imperative even if it requires implementing it by way of telemedicine (16).
The Gastroenterology, Hepatology and Pediatric Nutrition Unit of Gastronutriped, IPS in Bogota has diagnosed, followed up and supported children suffering from CF in Colombia since 1966. Because of the appearance of food allergies in some of these patients, we developed a growing interest in collecting all reliable data on patients with both ailments in order to determine the prevalence of this combination in our unit.
METHODOLOGY
This is a retrospective study of male and female patients diagnosed with CF and treated at the Unit of Gastroenterology, Hepatology and Nutrition of Gastronutriped IPS between 2009 and 2013. Patients ages ranged from newborns to 18 years old. Patients with malabsorption syndrome, etiologies other than CF, metabolic syndrome related to CFTR, and unconfirmed illnesses were excluded.
MATERIALS AND METHODS
Digital and paper medical records and nursing registry entries between the years 2009 and 2013 for patients diagnosed with CF and registered in Gastronutripeds data base were reviewed.
The following variables were taken into consideration: age at diagnosis, initial symptoms, electrolyte values in sweat, acid steatocrit (These measurements were taken during follow-ups except for one which was documented at the first instance,), fecal elastase, genetic testing for cystic fibrosis and data from other studies such as hepatobiliary ultrasound, abdominal CT scans, initial diagnoses, pulmonary involvement, pancreatic involvement, hepatobiliary involvement, enzyme replacement therapy, food allergy diagnoses, type of allergy, endoscopy, pathological studies, skin tests and ImmunoCAP testing. The data obtained was entered into a Microsoft Excel database for further analysis and investigation.
RESULTS
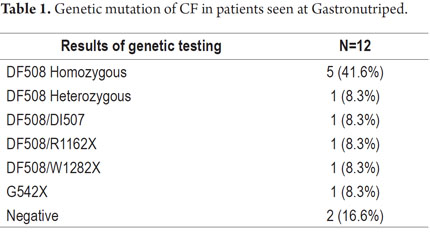
Records of 30 patients from 2009 to the present were found. The illness was ruled out in one case, another diagnosis remains unfinished, and one patient has a metabolic syndrome related to CFTR. Hence, the final sample included 27 patients (Table 1).
The primary initial diagnosis was CF in 70.3% of the patients (19/27). 77.7% (N=21/27) of the group presented pulmonary involvement, 100% presented pancreatic involvement and 62.9% (N=17/27) have some degree of liver involvement.
Age at the time of diagnosis of CF was known for 20 of the 27 patients. The minimum age at diagnosis was less than one month and the maximum age at diagnosis was 13 years with an average age 3.6 years.
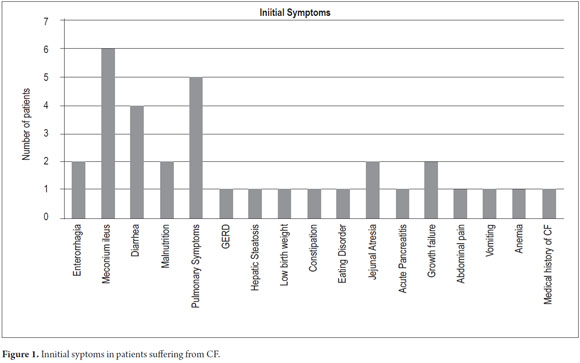
Although initial symptoms were manifold, 11 (42%) of the 27 children had no record of initial symptoms. Four (4) patients were monosymptomatic while the rest reported two or three symptoms. The most frequent symptom was rectal bleeding followed by meconium ileus and malnutrition (Figure 1).
Results from iontophoresis, the gold standard of pathology tests, were found for 15 patients (N=15/27, 55%) 13 of whom tested positive 86.6% (N=13/15). For 11 patients (N=11/27, 40.7%) records of a second test were found. Of these nine patients 81.8% (N=9/11) tested positive. Because of the diagnostic protocols for this pathology, it is more than feasible that 100% of the children may have had one or two iontophoresis tests. For this reason, we consider that this test was under recorded.
Out of 27 patients, only 12 had records of genetic testing. The most frequent mutation was Delta F508 which was found in 10 of the 12 patients (83.2%).
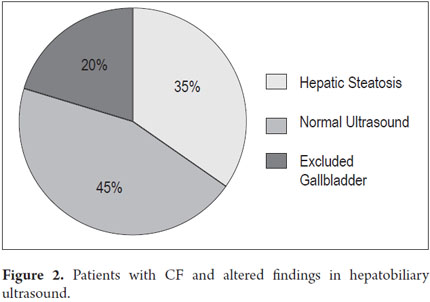
Acid steatocrit data was obtained for 18 patients. There were normal values in 83.3% (15/18) of the sub-sample. All but one were part of a follow-up. Figures for fecal pancreatic elastase which is a first rate indirect test of pancreatic exocrine insufficiency were found for four patients. Levels recorded were <15, 140, 197, <100µg/g while the normal reference level is >200µg/g. Hepatobiliary sonograms were found in the medical records of 21 of the 27 patients. Findings ranged between normal and hepatic steatosis (Figure 2).
Among the 27 patients with confirmed CF diagnoses, four presented concomitant food allergies. This represents a prevalence of 14.8% in four years of monitoring. The clinical expressions of CF and food allergies included one case of eosinophilic proctocolitis, one case of atopic dermatitis due to allergy to cows milk protein, one case of atopic dermatitis that tested positive with RAST for egg whites, oranges, avocados, pears and fish, and one case of enteropathy associated with an allergy to cows milk protein.
DISCUSSION
Literature on cystic fibrosis concomitant with food allergies is scarce. A 1994 study by Lucarelli et al. is the oldest article found in our search (11). It described 20 patients who had recently been diagnosed with CF who had persistent gastrointestinal symptoms and/or whose nutritional status did not improve despite adequate diet and enzymatic supplement. As a result, it was suspected that these were cases of CF associated with food allergies. A diet that eliminated cows milk and egg proteins was tried for 4 to 6 weeks. It resulted in improvement in the gastrointestinal symptomatology especially in reducing diarrhea and beginning weight gain. The reintroduction of the allergens was related to relapses of the symptoms (11). Unfortunately, the article did not specify whether endoscopic studies had been performed nor did it specify the type of clinical expression of the food allergies.
In 2013, Goralsky published a report about 3 patients (7). The first was a 15 year old female patient who had been diagnosed with CF and pancreatic insufficiency. Although her nutritional status was good, her abdominal pain, nausea and periprandial vomiting persisted despite treatment with proton pump inhibitors (PPIs). She was diagnosed with cholelithiasis and subsequently underwent a laparoscopic cholecystectomy, but her symptoms did not improve. She began to lose weight, so additional studies were done. An esophagogastroduodenoscopy and subsequent pathological tests showed an eosinophil count of 35 per field which was consistent with eosinophilic esophagitis. This was treated with budesonide which led to a resolution of her symptoms.
The second case was a 4 year old girl with pancreatic insufficiency, poor nutritional status and clinical suspicion of gastroesophageal reflux disease. She was treated with PPIs but developed a severe aversion to food related to severe dysphagia. Macroscopic findings from an esophagogastroduodenoscopy were compatible with eosinophilic esophagitis, and the pathology report showed more than 50 eosinophils per field. Subsequently allergies to soy, peas and grass were confirmed and treated with budesonide and elemental formula.
The final patient was a 12 year old boy suffering from CF who had characteristics that were similar to the other two cases: recurring abdominal pain, poor food intake, and persistence of the symptoms in spite of treatment with PPIs. The pathology report following an esophagogastroduodenoscopy revealed 40 eosinophils per field with evidence of allergies to peanuts, eggs, soy, wheat and corn. The patient was treated with budesonide and elemental formula which led to an improvement of his symptoms.
The studys conclusion emphasizes the importance of performing differential diagnosis in patients with CF whose development is torpid. These authors study is the first formal report of a relation between CF and food allergies (7).
CF and food allergies are two entities with different physiopathology mechanisms which suggests that the pathogenesis of CF does not superimpose over food allergies even though they may be simultaneously present in the same individual. Since the overall prevalence of atopic disease has increased in the general population, food allergies should be considered when there are documented manifestations of atopy in any patient with CF especially when gastrointestinal symptoms persist after diagnoses have been ruled out (7). The complexity of both diagnoses and the presence of unspecified gastrointestinal symptoms which may be common to both entities must always be considered in deferential diagnosis and should never lead to their exclusion because, as it has been shown, they may coexist.
As outlined above, it is feasible for a patient to suffer from a chronic inherited disease with multisystemic involvement while simultaneously having other coexisting gastrointestinal entities whose pathophysiological mechanism is independent. Similarly, concomitant immune-based comorbidities such as dermatitis herpetiformis (DH) and celiac disease that condition inflammatory responses have been observed. The latter, involves complex interrelations between autoimmune factors such as the predisposition of the human leukocyte antigen (HLA), HLA-DQ2 and DQ8, genetics and the environment. In addition, pathogenic autoantibodies in both celiac disease and DH are predominantly of the IgA class with the IgG type gaining importance when there is a deficiency of the former (19).
We found that in the Gastronutriped sample of 27 CF patients from 2009 to the present, the prevalence of food allergies was 14.8% (4/27 patients). Those food allergies had diverse clinical expressions.
The patients recorded in Gastronutriped were treated according to international guidelines and with the experience gained during years of working with patients who have had both fibrosis and food allergies. We have obtained positive results in the resolution of symptoms and in patient weight gain. These types of entities, whether they are present separately or together, represent a challenge for the clinician, the family and the patient especially because of the complex treatment and strict monitoring that is required.
For patients suffering from CF, it is necessary to research, confirm and discard other entities such as food allergies. This is particularly important for those patients whose gastrointestinal symptoms do not improve depstie adequate handling and adherence to treatment.
CONCLUSION
It is crucial to suspect other pathologies in any patient suffering from CF when gastrointestinal symptoms persist despite adequate treatment and treatment adherence. Other clinical entities such as food allergies should be considered. This requires clinical diagnosis and confirmation with high and low endoscopy, measurement of specific IgE for allergens, skin tests and/or allergen challenges. Although the treatment for food allergies depends on clinical expression, in the majority of the cases it only implies elimination of allergens from the diet. This treatment, if correct, will contribute to improvement of gastrointestinal symptoms, and even skin and respiratory symptoms, and will positively affect the patients health and quality of life.
REFERENCES
1. Gaskin K. Exocrine Pancreatic Dysfunction Cystic Fibrosis. En Walker A, Goulet O, Kleinman R, Sherman P, Shneider B, Sanderson I. Pediatric Gastrointestinal Disease. Chapter 65. Fourth Edition. Editorial BC Decker; 2008. p. 1607-1623. [ Links ]
2. Salvatore D, Buzzetti R, Baldo E, Furnari ML, Lucidi V, Manunza D, et al. An overview of international literature from cystic fibrosis registries. Part 4: Update 2011. Journal of Cystic Fibrosis 2012; 12: 11: 480-493. [ Links ]
3. Borowitz D, Robinson K, Rosenfeld M, et al. Cystic Fibrosis Foundation Evidence-Based Guidelines for Management of Infants with Cystic Fibrosis. J Pediatr 2009; 155: S73-93. [ Links ]
4. Fiocchi A, Schünemann HJ, Brozek J, Restani P, Beyer K, Troncone R, et al. Diagnosis and Rationale for Action against Cows Milk Allergy (DRACMA). J Allergy Clin Immunol 2010; 3: 57-161. [ Links ]
5. Lobo J, Rojas-Balcazar JM, Noone PG. Recent Advances in Cystic Fibrosis. Clin Chest Med 2012; 33(2): 307-328. [ Links ]
6. O´Sullivan B, Freedman S. Cystic Fibrosis. Lancet 2009; 373: 1891-904. [ Links ]
7. Goralski JL, Lercher DM, Davis SD, Dellon ES. Eosinophilic esophagitis in cystic fibrosis: A case series and review of the literature. Journal of Cystic Fibrosis 2013; 12(1): 9-14. [ Links ]
8. Babinski D, Trawinska M. Rhinosinusitis in cystic fibrosis: Not a simple story. International Journal of Pediatric Otorhinolaryngology 2008; 72: 619-624. [ Links ]
9. Vásquez C, Hernández J, Barón O, Medina M, Dueñas E. Fibrosis quística [Monografía en Internet]. Colombia. Perspectiva Neumológica Boletín trimestral de la Fundación Neumológica Colombiana. 2011; 11(1). Disponible en: http:// www.neumologica.org/Archivos/perspectiva/PERS%20NEUMO% 20VOL%2011%20N%201.pdf [ Links ]
10. Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014; 133(2): 291-307.e5. [ Links ]
11. Lucarelli S, Quattrucci S, Zingoni AM, Frediani T, Diamanti S, Quintieri F, Barbato M, Cardi E. Food allergy in cystic fibrosis. Minerva Pediatrica 1994; 46(12): 543-548. [ Links ]
12. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol 2010; 125(2 suppl. 2): S116e25. [ Links ]
13. Zolkipli Q, Michaelis L, Roberts G. Diagnosis and management of food allergy. Paediatrics and child health 2012; 22 (7): 272-280. [ Links ]
14. Burney P, Keil T, Grabenhenrich L, Wong G. Chapter three - The Epidemiology of Food Allergy. In: Madsen CB, Crevel RWR, Mills C, Taylor SL, editors. Risk Management for Food Allergy San Diego: Academic Press; 2014. p. 45-64. [ Links ]
15. Fernández-Rivas M, Asero R. Chapter two - Which Foods Cause Food Allergy and How Is Food Allergy Treated? In: Madsen CB, Crevel RWR, Mills C, Taylor SL, editors. Risk Management for Food Allergy San Diego: Academic Press; 2014. p. 25-43. [ Links ]
16. Piazza-Waggoner C, et al. Case Study: Providing Evidence-Based Behavioral and Nutrition Treatment to a Toddler with Cystic Fibrosis and Multiple Food Allergies via Tele health. Pediatric Pulmonology 2006; 41: 1001-1004. [ Links ]
17. Niño R, Daza W, Dadán S. Electrolitos en sudor en pacientes pediátricos con fibrosis quística atendidos en la Unidad de Gastroenterología, Hepatología y Nutrición Pediátrica (Gastronutriped) de Bogotá. Tesis para optar al grado de Especialista en Pediatría, Universidad El Bosque 2009. [ Links ]
18. Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, et al. Diagnostic Approach and Management of Cows-Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines. Journal of Pediatric Gastroenterology & Nutrition 2012; 55: 221-229. [ Links ]
19. Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis: Part I. Epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol 2011 6; 64(6): 1017-1024. [ Links ]











 texto en
texto en