Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.3 Bogotá set. 2014
Case Report and Literature Review: Shortened 16 Week Treatment of Patient with Genotype 2 Hepatitis C Infection
Rolando J. Ortega Q. MD. (1), Mario Moscote G. MD. (2), Moisés Diago M. MD. (3)
(1) Internist, Gastroenterologist, Hepatologist, Director of the Division of Hepatology and Adjunct Director of the Division of Gastroenterology at the Clínica General del Norte in Barranquilla, Colombia. Institute for Biomedical Research in the Faculty of Health Sciences at Universidad de San Buenaventura in Cartagena, Colombia. E-mail: rolandoortegaquiroz@gmail.com
(2) Internist, Gastroenterologist and Director of the Division of Gastroenterology Division Director at the Clínica General del Norte. Barranquilla, Colombia.
(3) Internist, Gastroenterologist and Hepatologist. Chief of the Digestive Pathologies Division and Director of the Hepatology Service at the Manager Hepatology Hospital General de Valencia in Valencia, Spain.. E-mail: moisesdiagom@gmail.com
Received: 10-03-14 Accepted: 21-07-14
Abstract
We report the case of a 46-year patient infected with genotype 2 of hepatitis C. The patient received short-course treatment with low doses of pegylated interferon alfa 2b (1 mcg/week SC k) and ribavirin (800 mg/day orally) for 16 weeks. The patient had sustained virologic response at 12 weeks (SVR12) viral and at 24 weeks (SVR24). Tolerance to treatment was very good, and there were no clinically significant signs of anemia or adverse effects. We propose that this type of therapy be considered for patients with factors associated with good prognoses such as low viral loads, low levels of fibrosis (<F2), rapid viral response (RVR) and the IL28B CC genotype. This strategy can significantly reduce the costs associated with the new direct-acting antiviral agents (DAAs) of the Sofosbuvir type associated with Ribavirin. These should be administered for 12 weeks.
Keywords
Hepatitis C, genotype 2, shortened dual therapy, rapid viral response.
INTRODUCTION
Chronic Hepatitis C infections affect an estimated 160 million people. Hepatitis C virus (HCV) is one of the main causes of chronic hepatitis and cirrhosis, and is the principal etiology of hepatocellular carcinoma (HC) in the West. It is the most important indication for a liver transplant (1, 2). The mechanisms by which the virus infects the hepatocytes and reproduces itself have been partially clarified and have recently been the subject of extended review (3). Because of the marked heterogeneity in the HCV gene sequence, a system of genotype classifications has been established. There are currently seven genotypes, numbered from one to seven, based on major differences of 25-30%. Subtypes are defined by major differences of 15% and are in turn named with numbers and letters. There are at least 67 sub types (4). To date, no clear relation has been established between the genotype and the pathogenicity of the virus although the progression of fibrosis and hepatocarcinogenesis have been studied in detail (5). The current treatment for genotype 2 in our country is a combination of pegylated interferon alfa 2 a/b with ribavirin at a fixed-dose of 800 mg/day for 24 weeks. Treatment may be for a shorter time if a rapid virological response (RVR) is attained as measured by negative RNA at the fourth week of treatment (6). New direct-acting antivirals (DAAs) such as Sofosbuvir have recently been approved. When combined with Ribavirin, they can shorten therapy to 12 weeks with fewer adverse side-effects (7, 8). This study presents the clinical case of a patient with a genotype 2 hepatitis C infection who received dual therapy of pegylated interferon and ribavirin shortened to 16 weeks and who reached a rapid virological response (RVR) at 12 and 24 weeks.
CLINICAL CASE
A 46 year old female patient tested anti-HCV positive in May 2012 while donating blood to a blood bank. The patient denied any past transfusions, drug use, tattoos, piercing or any other known way that transmission could have occurred. She had had a caesarean section 20 years earlier (1), had had only one pregnancy and one and no miscarriages or abortions, had undergone curettage and a biopsy due to metrorrhagia 21 years earlier, and had used vitamin enriched serums with no specific motive 25 years earlier. She came to the Hepatology Service in February 2012, two months after receiving the HCV confirmation notice from the blood bank. Real-time quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR, TaqMan) with an index of sensitivity from 10 UI/ml showed her viral load to be 47,526 iu/ml, Log 4.68 and showed that she had a genotype 2 infection. Polymorphic study of IL 28B (rs 12979860) reported genotype CC. Transient elastography employing an Echosens FibroScan 502 was used to stage the development of the disease. It showed 4.4 kPa (10 valid measurements, IQR 0.3 which corresponds to FO Fibrosis.
Physical examination showed that the patient was in good overall condition with no signs of chronic hepatopathy. Her body mass index was 24.9. Blood tests showed hemoglobin level at 13.1 g/dl, leukocyte count: 4,810/mm3, neutrophil count: 2,410/mm3, platelet count: 330,000 mm3, AST: 22 IU/L, (30) ALT: 30 IU/L, (30) GGTP: 10 IU/L, (40) alkaline phosphatase: 92 IU/L, (206) TSH: 1.6 mIU/L, prothrombin time: 9 seconds, INR: 0.8, total bilirubin: 0.6 mg/dl, plasma glucose: 96 mg/dl, creatinine: 0.8 mg/dl, uric acid: 4.6 mg/dl, albumin: 4.2 g/dl, proteins: 7.1 g/dl. A pregnancy test was negative. An abdominal ultrasound was normal. The disease showed no evidence of fibrosis or inflammatory activity. The diseases stage was discussed with the patient.
Due to personal reasons and fear of the effects of the virus, the patient sought treatment. The possibility of dual therapy shortened to 16 weeks according to viral kinetics during treatment was proposed. Treatment with pegylated interferon alpha 2b and 800 mg of Ribavirin per day, divided into 2 doses every 12 hours began on May 31, 2012 with. The dosage of pegylated interferon alpha 2b was 60 mcg/week administered subcutaneously (1mcq/kg), and the dosage of ribavirin was 800 mg/per day. Viral kinetics showed a negative viral load in the second week. Results in the fourth, twelfth, and sixteenth week were all negative. Treatment was ended after sixteen weeks. Follow-up of sustained viral response (SVR) and healing showed negative results for the virus week 12 (SVR 12) and at week 24 (SVR 24). The patients tolerance to treatment was very good with mild flu-like symptoms which resolved in the first 4 weeks. Her hemoglobin dropped to 2 g/dl in total but with no repercussions on the patients daily activities. There were discrete decreases of neutrophils and platelets that reached low points at week 12 (1,940 mm3 and 268,000 mm3, respectively). 12 weeks after treatment, the complete blood count recovered initial values, AST was 16 IU/L, (30) and ALT was 20 IU/L (30).
DISCUSSION
Hepatitis C is a severe public health problem in every part of the world. Current evidence suggests that it is associated with a huge clinical and economic burden. The prevalence of cirrhosis related to hepatitis C has increased over the last decade, and it is projected to continue growing until it peaks in the next 10 to 15 years. The prevalence of hepatitis C in patients suffering from hepatocellular carcinoma ranges between 20% and 90%, and the relative risk that a patient will be infected patient is 25 times greater than that of the general population. Mortality rates related to liver disease are significantly higher in patients with chronic infections especially in people over 40 years of age. After transplants, recurrence of the virus with swift progression towards fibrosis, cirrhosis and decompensated cirrhosis is universal (40% within 5 years) (9). Hepatitis C is usually chronic, and spontaneous clearance of the virus only comes about in exceptional cases.
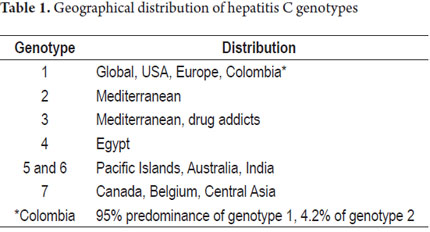
Genotype 1 is most prevalent in the USA (1a) and Europe (1b) where it accounts for 75% of cases. Genotype 2 prevails in the Mediterranean; genotype 3 mostly affects drug addict groups; genotype 4 has been isolated in Egypt; genotypes 5 and 6 have been found on Pacific islands and in India, Australia and New Zealand; and genotype 7 has been found in Canada, Belgium and Central Africa (2, 4). In Colombia, a series of 284 patients suffering from hepatitis C demonstrated a total prevalence of genotype 1 of 95% which breaks down into 16% prevalence of genotype 1a, 71% prevalence of genotype 1b, and an 8% prevalence of both genotypes 1a and 1b. The study also showed a 4.2% of prevalence of genotype 2 and a 0.7% prevalence of genotype 3 (Table 1) (10).
There is evidence that suggests that treatment of hepatitis is advisable because it is associated with improvements in patient quality of life, fibrosis, portal pressure, the risk of hepatocellular carcinoma, liver failure and mortality rates (11, 12).
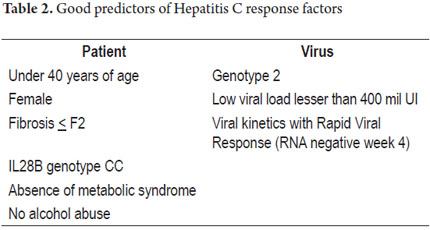
Patient factors associated with higher probabilities of reaching an SVR include: absence of fibrosis (F0), moderate fibrosis (F2), genotype 2, viral loads below 400,000 UI/ml, genotype CC of IL28B, absence of metabolic syndrome, being female, no alcohol abuse, and absence of HIV (Table 2) (2, 13, 14).
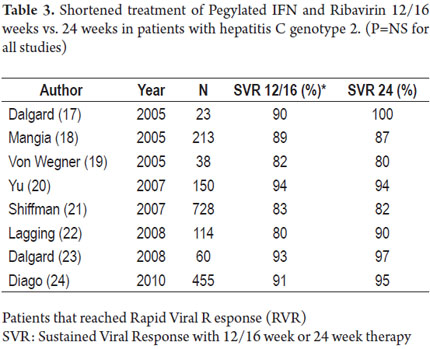
After the initial studies with pegylated interferon alpha 2b, it was noticed that there was no difference in responses between dosages of 1.5 mcg/kg/week and 1.0 mcg/kg/week (15). In addition, patients with genotypes 2 and 3 reached SVR with low dosages of Ribavirin (800mg/day) as well as with high dosages per weight of Ribavirin. They also reached SVR in shorter periods of up 24 weeks unlike patients with genotype 1 that required 48 weeks (16). The SVR rates in these patients stood between 75 to 80% which triggered a series of studies in order to evaluate reduced treatment periods and to check whether responses of genotypes 2 and 3 were indeed similar. With the results, it became evident that up to a 93% of patients with genotype 2 and viral loads below 400,000 UI/ml were reaching SVR with only 12-16 weeks of treatment if they were negative at week 4 indicating a rapid viral response (Table 3) (17-24).
In the ACCELERATE study of 1309 patients with genotypes 2 and 3 who were randomized into 16 week and 24 week treatment groups, 66% (863 patients, 458 after 16 weeks and 405 after 24 weeks) of the total study population reached rapid viral responses (RVR). The SVR in patients with viral loads of < 400,000 UI/ml was 91% in the 16 week group and 95% in the 24 week group (P=0, 20) which highlights the predictive value of RVR among low viral load patients (24). In another study, patients with genotype 3 showed less response to the treatment, possibly due to associated steatosis (25). Two metaanalyses of dual treatment for genotypes 2 and 3 which included 7 and 8 studies respectively concluded that overall SVR among patients with genotype 2 was 74% while for patients with genotype 3 it was 68%. O the patients with genotype 2 and rapid viral response (RVR), 60% to 85% of the cases reached SVR rates between 93% and 100%. No statistically significant differences between 12, 16 and 24 weeks of treatment were found (26, 27). In another study, reduction of the dose from 800 mg/day of Ribavirin to 400 mg/day did not affect the 24 week rate of SVR (28).
The incorporation of first generation protease inhibitors Boceprevir and Telaprevir in 2011, benefited only patients with genotype 1 whose SVR increased by 30% from 40% to a 70% (6, 7). Nevertheless a recent study has cast the usefulness of these medications in doubt. The study included 233 drug-naive patients with genotype 1, fibrosis < F3, and viral loads less than 600,000 iu/ml, separated into two groups of patients who reached RVR (48%) after a lead-in time. One of the groups continued with pegylated interferon alpha 2b and ribavirin for 24 weeks while the second group was given triple therapy with Boceprevir until week 28. Both groups reached 90% SVR by the twelfth week (29).
Recent and ongoing approvals of new direct-acting antivirals (DAA) that include nucleoside analog reverse-transcriptase inhibitors (NARTIs or NRTIs) (Sofosbuvir), NS5A inhibitors (daclatasvir, ledipasvir) and second-generation protease inhibitors (simeprevir, faldaprevir) open up new treatment and therapy options for patients with hepatitis C. Shorter, more efficient therapies, fewer tablets, no interactions, and a better safety profile mean that these drugs may become front-line responders in the near future (8). Electron, fusion, fission and neutrino studies with Sofosbuvir reveal excellent responses from patients infected with all genotypes of HCV including genotype 2. Twelve week treatment using a combination of sofosbuvir and ribavirin allows more than 93% of all patients with genotype 2, both drug naive and previous non-responders, to attain SVR. Response is reduced in previous non-responding patients with advanced fibrosis who need 16 weeks of treatment. Treating genotype 3 seems to be slightly more difficult since it must be handled with different schemes than those used for genotype 2. These schemes include using the sofosbuvir and ribavirin combination for 24 weeks and adding a third antiviral to the combination (30-32). Recently, the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) published their guides with suggestions based on newly released phase 3 studies. For the naive non-cirrhotic patients (< F3) with genotype 2 infections, the first treatment option is the combination of daily 400 mg doses of sofosbuvir and 1000-1200 mg doses of ribavirin for 12 weeks,. In patients with cirrhosis or no response to previous treatment, this therapy should be extended to 16 or 20 weeks. The combination of pegylated interferon, ribavirin and sofosbuvir for 12 weeks is an alternative (33, 34).
The costs of the new therapies remain to be defined. They will be approved country by country, taking into consideration the condition of the patient and the economic constraints of the health systems.
The conclusion from the case presented here is that treatment with low dosages of pegylated interferon and ribavirin for 16 weeks is an alternative for patients with genotype 2 who have good predictors of response factors such as fibrosis < F2, viral loads less than 400,000 iu/ml, rapid viral responses and genotype CC of IL28B. Because this treatment scheme has lower costs than the new antivirals, this is especially important for patients who wish to be treated before reaching advanced stages of fibrosis and who have good tolerance to the medication.
Acknowledgement
We would like to acknowledge and thank Angie Lopez, an outpatient nurse in the Hepatology department of the Clinica General del Norte in Barranquilla.
REFERENCES
1. Blachier M, Leleu H, Peck-Radosavljevic M, et al. The burden of liver disease in Europe: A review of available epidemiological data. J Hepatol 2013; 58: 593-608. [ Links ]
2. Rosen H. Chronic hepatitis C infection. N Eng J Med 2011; 364: 2429-2438. [ Links ]
3. Polyak S, Morishima Ch, Scott J, et al. A summary of the 18th international symposium on hepatitis C virus and related virus. Gastroenterology 2012; 142: e1-e5. [ Links ]
4. Smith D, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resourse. Hepatology 2014; 59: 318-327. [ Links ]
5. Asselah T, Biéche I, Sabbagh A, et al. Gene expression and hepatitis C virus infection. Gut 2009; 58: 846-858. [ Links ]
6. Jake Liang T, Ghany M. Current and future therapies for hepatitis C virus infection. N Eng J Med 2013; 368: 1907-1917. [ Links ]
7. Jesudian A, De Jong Y, Jacobson I. Emerging therapeutic for hepatitis C virus infection. Clin Gastroenterol Hepatol 2013; 11: 612-619. [ Links ]
8. Mariño Z, Van Bommel F, Forns X, et al. New concepts of sofosbuvir-based treatment regimens in patients with hepatitis C. Gut 2014; 63: 207-215. [ Links ]
9. Younossi Z, Fanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther 2014; 39: 518-531. [ Links ]
10. Arias Y, Echeverri J, Castro M, et al. Frecuencia de genotipos y subtipos de virus de la hepatitis C en pacientes Colombianos con infección crónica. Rev Médica Sanitas 2010; 13: 10-19. [ Links ]
11. Van der Meer A, Wedemeyer H, Feld J, et al. Is there sufficient evidence to recommend antiviral therapy in hepatitis C. J Hepatol 2014; 60: 191-196. [ Links ]
12. Chou R, Hartung D, Rahaman B, et al. Comparative effectiveness of antiviral treatment for hepatitis C virus infection in adults: A systematic review. Ann Inter Med 2013; 158: 114-123. [ Links ]
13. Nelson Hayes C, Imamura M, Aikata H, et al. Genetics of IL28B and HCV response to infection and treatment. Nat Rev Gastroenterol Hepatol 2012; 9: 406-417. [ Links ]
14. Ge D, Fellay J, Thompson A, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 46: 399-401. [ Links ]
15. Hoofnagle J, Seef L. Peginterferon and Ribavirin for chronic hepatitis C. N Eng J Med 2006; 355: 2444-2451. [ Links ]
16. Hadziyannis S, Sette H, Morgan T, et al. Peginterferon alfa 2a and ribavirin combination therapy in chronic hepatitis C. Ann Int Med 2004; 140: 346-355. [ Links ]
17. Dalgard O, Bjoro K, Hellum KB, et al. Treatment with pegylated interferon and ribavirin in HCV infection with genotype 2 or 3 for 14 weeks: A pilot study. Hepatology 2004; 40: 1260-1265. [ Links ]
18. Mangia A, Sontoro R, Minerva N, et al. Peginterferon alfa 2b and ribavirin for 12 vs 24 weeks in HCV genotype 2 or 3. N Eng J Med 2005; 352: 2609-2617. [ Links ]
19. Von Wagner M, Huber M, Berg T, et al. Pegiterferon alfa 2a and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology 2005; 129: 522-527. [ Links ]
20. Lu ML, Dai Ch, Huang JF, et al. A randomized study of peginterferon and ribavirin for 16 vs 24 weeks in patients genotype 2 chronic hepatitis C. Gut 2007; 56: 553-559. [ Links ]
21. Shiffman M, Suter F, Bacon B, et al. Peginterferon alfa 2a and ribavirin for 16 and 24 weeks in HCV genotype 2 or 3. N Eng J Med 2007; 357: 124-134. [ Links ]
22. Lagging M, Langeland N, Pedersen C, et al. Randomized comparison of 12 or 24 weeks of peginterferon alfa 2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection. Hepatology 2008; 47: 1837-1845. [ Links ]
23. Dalgard O, Bjoro K, Ring-Larsen H, et al. Pegilated interferon alfa and ribavirin for 14 vs 24 weeks in patients hepatitis C genotype 2 or 3 and rapid virological response. Hepatology 2008; 47: 35-43. [ Links ]
24. Diago M, Shiffman M, Bronowicki JP, et al. Identifying hepatitis C virus genotype 2/3 patients who can receive a 16 week abbreviated course of Peginterferon alfa 2a (40 KD) plus ribavirin. Hepatology 2010; 51: 1897-1903. [ Links ]
25. Zeuzem S, Hultcrantz R, Bourliere M, et al. Peginterferon alfa 2b plus ribavirin for treatment chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol 2004; 40: 993-999. [ Links ]
26. Andriulli A, Mangia A, Iacobellis A, et al. Meta-analysis: the outcome of antiviral therapy in HCV genotype 2 and 3 infected patients with chronic hepatitis. Aliment Pharmacol Ther 2008; 28: 397-404. [ Links ]
27. Romero-Gómez M, Lacalle Remigio JR. Tratamiento de la hepatitis C por genotipos 2 y 3: revisión sistemática. Gastroenterol Hepatol 2006; 29: 139-145. [ Links ]
28. Ferenci P, Brunner H, Laferl H, et al. A randomized, prospective trial ribavirin 400 mg/day vs. 800 mg/day in combination with peginterferon alfa 2a in hepatitis C virus genotype 2 or 3. Hepatology 2008; 47: 1816-1823. [ Links ]
29. Pearlman B, Ehleben C. Hepatitis C genotype 1 virus with low viral load and rapid virological response to peginterferon/ribavirin obviates a protease inhibitor. Hepatology 2014; 59: 71-77. [ Links ]
30. Gane E, Stedman C, Hyland R, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C (Electron). N Eng J Med 2013; 368: 34-44. [ Links ]
31. Jacobson I, Gordon S, Kowdley K, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options (Positron-Fusion). N Eng J Med 2013; 368: 1867-1877. [ Links ]
32. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection (Fission-Neutrino). N Eng J Med 2013; 368: 878-887. [ Links ]
33. Pawlotsky JM, Aghemo A, Dusheiko G, et al. EASL clinical practice guidelines 2014: management of hepatitis C virus infection. www.easl.eu. [ Links ]
34. AASLD-IDSA Hepatitis C Guidelines 2014. Recommen-dations for testing, managing and treating hepatitis C. www.hcvguidelines.org. [ Links ]











 texto en
texto en