Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.4 Bogotá oct./dic. 2017
https://doi.org/10.22516/25007440.174
Original articles
The Role of Biosimilars in Inflammatory Bowel Disease: A Reality in our Country
1 Coloproctólogo de la Universidad Militar Nueva Granada, Hospital Militar Central; director científico del Instituto de Coloproctología ICO S.A.S. Clínica Las Américas, Medellín, Colombia. Correspondencia: juanmarquezv@gmail.com
2 Jefe de la unidad de enfermedad inflamatoria intestinal, profesor de medicina Hospital Clínico Universitario Lozano Blesa, Facultad de Medicina IIS Aragón; Centro de Investigación Biomédica en Red en el Área temática de Enfermedades Hepáticas (CIBEREHD). Zaragoza, España
The term Inflammatory Bowel Disease (IBD) is used to identify several entities, the two most important of which are Idiopathic Ulcerative Colitis (IUC) and Crohn’s Disease (EC). Both are multifactorial in origin, are chronic and recurrent, are characterized by inflammation, have varying degrees of severity, and potentially involve other organs.
In the last decade there has been renewed interest in these entities due to growing incidence and to the development of drugs called biologicals. For the first time, these drugs have changed the natural history of these diseases. They are produced or derived from living organisms and represent the fastest growing sector in the global pharmaceutical industry. Due to commercial interest, products similar but not identical to the original products have been developed and submitted to regulatory authorities.
The European Medicines Agency has approved the first biosimilar for infliximab for all indications for which it has been approved. These include moderate to severe ulcerative colitis (UC) at all ages, moderate to severe Crohn’s disease (CD) at all ages, fistulizing Crohn’s disease, Psoriasic arthritis, Psoriasis, Ankylosing Spondylitis, and Rheumatoid Arthritis 1.
In this review, we define what these products are, as well as some concepts coined for their usage including extrapolation, substitution and interchangeability. We also make a historical review of their development, current indications, the positions of various scientific associations with respect to them, and - most importantly - provide real-life data regarding their effectiveness, safety and costs in countries where they have been used.
Key words: Biosimilar; biological agents; anti TNF; extrapolation; substitution and interchangeability
La enfermedad inflamatoria intestinal (EII) es un término con el que se conocen varias entidades, las 2 más importantes son: la colitis ulcerativa idiopática (CUI) y la enfermedad de Crohn (EC), cuyo origen es multifactorial y se caracterizan por un fenómeno inflamatorio, crónico, recurrente con diferentes grados de severidad del tubo digestivo; pero además con afectación potencial de otros órganos.
En la última década ha habido un renovado interés en dichas entidades, debido a una incidencia creciente de estas, pero también debido al desarrollo de medicamentos que por primera vez están cambiando la historia natural de estas enfermedades: son los medicamentos llamados biológicos, que son aquellos producidos o derivados de organismos vivos y representan el sector de mayor crecimiento en la industria farmacéutica mundial. Debido al interés comercial, se han desarrollado y sometido a las autoridades reguladoras productos no originadores; similares, pero no idénticos a los productos de referencia.
La Agencia Europea de Medicamentos (EMA) aprobó el primer biosimilar del infliximab para todas las indicaciones en las cuales este estaba aprobado, incluidas: colitis ulcerativa moderada a severa en todas las edades, EC moderada a severa en todas las edades, al igual que EC fistulizante, artritis psoriásica (AP), psoriasis, espondilitis anquilosante (EA) y artritis reumatoide (AR) 1.
En esta revisión nos proponemos definir lo que son estos productos al igual que algunos conceptos relacionados (extrapolación, sustitución e intercambiabilidad), además hacer una revisión histórica de su desarrollo, sus indicaciones actuales, las posiciones de las diferentes asociaciones científicas al respecto y, lo más importante, brindar datos de la vida real en cuanto a su efectividad, seguridad y costos en los diferentes países donde se han estado usando.
Palabras clave: Biosimilares; agentes biológicos; anti-TNF; extrapolación, sustitución e intercambiabilidad
Biological medicines, produced or derived from living organisms, represent the fastest growing sector in the global pharmaceutical industry. In 2007, global pharmaceutical industry sales of biologicals was around 100 billion dollars, but in just 10 years since, in 2017 sales of these products are estimated to be 20% of the total global pharmaceutical market, and by 2020 they will amount to 250 billion dollars. 2 In 2008, globally two of the top ten medicines sold were biologicals, but by 2016 seven of the top ten were biologicals. The number of biologicals approved by the US Food and Drug Administration (FDA) has risen 33% to 60% every year while the United States consumes 50% of world sales. 3
In 2012, eight of the ten drugs not included in the Colombian health benefit plan that cost the Colombian government the most from repayments were of biological origin. In 2013, biologicals accounted for approximately 36% (USD $ 1.25 billion) of the total Colombian pharmaceutical market. 4
Due to commercial interest, biosimilar products have been developed and submitted to the regulatory authorities. Biosimilars are not identical to the originator biological products because these are large, complex molecular structures and the variability inherent in both manufacturing process and in the very nature of a biological makes it impossible to create an identical molecule. Among other contributing causes are expiration of patents of the first biological pioneers, the global financial crisis which has resulted in significant reductions in sustainable health expenses, and the estimated cost of this type of medicine which is between USD $50,000 and $ 250,000 per patient per year. This is why IMS Health calculates that by 2020 biosimilar sales will be between USD $10 billion and USD $25 billion. 5
The difference in the cost of producing a generic (which is calculated to be between 1 and 4 million dollars) with that of a biological that manages to become commercial (only 10% achieve commercial viability) is around two billion dollars. Biosimilars cost between 100 and 250 million dollars to produce and take seven to eight years before they can be sold commercially, but it is estimated that their profitability is very high. Remicade® earned USD $9.2 billion globally for all indications while Humira® earned 13 billion dollars in 2014. 6 This is true for the first biosimilars that are competing with biological originator s because, since price is really their only commercial argument, every time a new one enters the market, the milligram-to-milligram value of the originator and its descendants must fall in order to remain competitive. Consequently, profits fall for both producers and wholesalers to the point that the investment is no longer be profitable.
In 2005 the European Medicines Agency (EMA) defined biological, biologically similar and bioequivalent to standardize the basic characteristics of biological drugs. 7
Biologicals are medicines whose active principle is a biological substance such as recombinant DNA, an attenuated virus, blood and plasma derivatives, and monoclonal antibodies produced or extracted from a physical and chemically characterized biological source.
New generation biologicals are state-of-the-art agents whose therapeutic targets or biological targets are the same as the reference product but which are elaborated differently and individually as new products and separately undergo conventional efficacy and safety tests. 8
Biosimilars are medicines developed by another laboratory which maintain that the new agent is similar to the originator biological product or reference biological medicine which had been approved by regulatory agencies and marketed. A biosimilar contains the same active component, is designed to be used against the same disease, and uses the same route of administration at the same doses as the reference medicine.
In 2005, EMA coined the term “biosimilars” and designed regulations for these drugs. To date, it has approved sixteen biosimilars. 9 The first approved anywhere in the world was a biosimilar of somatropin (recombinant human growth hormone) which was authorized for sale by the EMA in April 2006. 10
The day “everything changed” with respect to inflammatory bowel disease (IBD) was September 13, 2013 when the EMA took the final step in the approval of the first infliximab (IFX) biosimilar. CT-P13 (Inflectra®/Remsima® Celltrion Healthcare , Korea) was approved for all indications for which IFX had already been approved including moderate to severe ulcerative colitis (UC) at all ages, moderate to severe Crohn’s Disease (CD) at all ages, fistulizing CD, psoriatic arthritis, psoriasis, ankylosing spondylitis and rheumatoid arthritis. 1
European approval for these indications was based on recognition that the primary mechanism of action of IFX is the same in all of these entities and that the information provided by non-clinical comprehensive studies showed that the main physicochemical characteristics and biological activity of CT -P13 and reference drug were comparable. 11
EMA approval was based on only two studies: the PLANETAS study of ankylosing spondylitis and the PLANETRA study of rheumatoid arthritis. 12 PLANETAS was a Phase I study that included 250 patients with AS. It demonstrated bioequivalence between CT-P13 and IFX in terms of pharmacokinetics. The clinical response at weeks 14 was 63% for CT-P13 and 65% for Remicade®. At 30 weeks clinical responses were 71% for CT-P13 and 72% for Remicade®. Antibodies to infliximab (Active Pharmaceutical Ingredient) were present in 9% of patients treated with CT-P13 at 14 weeks and in 27% of these patients at 30 weeks. For patients treated with Remicade®, antibodies were present in 11% and 23% of at weeks 14 and 30, respectively. Reactions to infusions at week 30 affected 65% of the CT-P13 group and 64% of the Remicade® group. These factors all maintained equivalency until week 54. (13
PLANETRA was phase III study of 606 patients with rheumatoid arthritis who were grouped randomly to receive 3 mg/kg of CT-P13 (302 patients) or IFX (304 patients) at weeks 0, 2, and 6. After week six the same dosages of the same drugs were administered with the addition of methotrexate every eight weeks. The primary objective was to evaluate therapeutic equivalence with a 95% confidence interval (CI). 14
After this approval, the economic impact was immediate: the revenues of MSD, which markets Remicade® in Europe, fell from US $ 2.3 billion in 2014 to US $ 1.8 billion in 2015 following introduction of the biosimilar into the market. 6
In May 2016, SB2 (Flixabi®, Samsung Bioepsis, South Korea) became the second IFX biosimilar authorized by EMA. It was approved for sale in Europe for treatment of moderate to severe UC, CD, PA, AS, and RA. The approval was based on clinical data from both a randomized phase I pharmacokinetic study that compared SB2 and Remicade® in 159 healthy individuals and a double-blind, multinational, randomized, parallel group phase III study that compared SB2 to the reference IFX in 584 patients with moderate to severe AR despite therapy with methotrexate. 10
On February 9, 2016, the FDA’s Arthritis Advisory Committee met to study approval of CT-P13 in the United States. The decision of this committee followed exactly the same guidelines that led the EMA to approve CT-P13 in Europe. The results of the studies were extrapolated to include all of indications for which the IFX is used, and the use of CT-P13 was authorized en bloc for all of these indications rather than individually authorizing use indication by indication. A clinical study was never presented for ulcerative colitis or Crohn’s Disease as support for approval of the biosimilar’s use to treat these indications. On April 5, 2016, the FDA approved CT-P13 for treatment of all indications for IFX (Remicade®). The decision was based on the same studies used by the EMA which demonstrated biosimilarity to the originator compound through the use of comparisons of structure, function, animal toxicity, human pharmacokinetics and pharmacodynamics, clinical efficacy, safety and immunogenicity with a calculation of a 90% CI. The recommendation of the committee was accepted. On July 12, 2016, the FDA’s arthritis advisory committee recommended that ABP 501, the adalimumab biosimilar (ADA) (Humira®) from Amgen (Thousand Oaks, California, United States), be licensed for use. Based on extrapolation of a phase III clinical study of AR and a Phase III clinical study of plaque psoriasis, the licensing recommendation extended to all indications for which Humira® is currently prescribed. 12. Currently, more than 20 IFX and ADA biosimilars are being developed.
Although a clinician would consider that biosimilarity should be determined more as a function of efficacy and safety, for the FDA and the EMA, it is more important to demonstrate analytical similarity between the proposed product and the reference product that it is to independently establish the characteristics listed above. Even in our midst, this became a reality when President Juan Manuel Santos Calderón approved decree number 1782 of 2014 of the Ministry of Health and Social Protection on September 18, 2014. 15 The decree authorizes the use of biosimilars in our country through the abbreviated route of comparability. 4 Prior to signing the decree, Santos had received political pressures from the US government in a contrary direction (see the letter from Vice President Biden, Annex 1).
All of the above is very topical and controversial due to the therapeutic and economic implications that are involved. This is especially so since, according to the evidence provided and duly approved, these medications are no less effective or less safe than the originator s, therefore, the main argument and support for allowing them to be sold is cost savings.
Definitions
A biotechnological drug, or biological, is defined as a pharmaceutical agent whose active principle is a biological substance such as recombinant DNA, an attenuated virus, blood or plasma derivatives, and monoclonal antibodies that are produced or extracted from a biological source (e.g. proteins derived from DNA). They are physically and chemically characterized by processes of hybridization and recombination that require living organisms for their production, and their aim is to act on the immune system. 16 In an inflammatory process, various cell groups and mediators of inflammation which are susceptible to therapeutic action are involved. These are called therapeutic targets (biological targets).
Inhibition of tumor necrosis factor alpha (TNF-α) has been shown to be effective for controlling moderate to severe inflammation in CD and UC, as well as in other immune inflammatory disorders such as AS, psoriasis and AR. This has caused a revolution in the management of IBD patients and patients with fistulizing damage due to CD. Before the advent of this class of drugs two decades ago, but after the Targan et al. study, 17 there was no effective therapy for treating these patients. 18 Steroids induce remission, but are not indicated for the maintenance phase while immunomodulators act to prevent relapse, but not during outbreaks. The advantage of biologicals is that they are as effective for inducing remission as they are for maintenance but do not have the deleterious effects of steroids.
Anti-TNF-α therapy has had great achievements: for the first time sustained deep remission defined as clinical remission, free of steroids, with negative biomarkers and no mucosal scarring is being achieved in cases of moderate to severe IBD. This translates into prevention of structural damage and reduction of hospitalization time and need for surgery and ultimately results in lower morbidity and mortality rates and improvement in patient quality of life.18,19,20
At present, three types of inhibitors or antagonists of TNF-α are available in Colombia: specific monoclonal antibodies including IFX, ADA and golimumab for ulcerative colitis; a biosimilar for IFX, CT-P13; and Remsima® (Celltrion, South Korea) for clinical use in the treatment of IBD. Biosimilars have been developed for ADA by Amgen (United States) but have not yet been authorized in our country.
Development of biosimilars is complex and expensive. For one to come to market, three conditions must be met: 9
A pharmaceutical company must find a biological that is interesting from the economic point of view and whose patent is about to expire.
The company must undertakes the whole development process including the risks and costs of producing a biosimilar.
A regulatory agency must approve the biosimilar.
There are different positions among the regulatory agencies to the point that they do not even have a consensus on how these drugs should be called. The FDA initially called them follow-on protein products although most recently the FDA calls them biosimilars. Health Canada calls them subsequent entry biologicals, and the EMA calls them biosimilars.
In Colombia, the term biosimilar is best known and for this reason is used in this article. Biocomparable is used in Mexico, the country with the largest number of Spanish-speaking people in the world.
The problem with these definitions is the term “similar”. Before arriving at this concept, a drug must undergo multiple in-vitro studies for tolerability, purity, safety, bioequivalence, effectiveness and pharmacokinetics and in vivo tests including randomized double-blind controlled trials with adequate power. After all of this, the term biosimilar was coined. 16 Nevertheless, for marketing purposes, laboratories must demonstrate that their product is similar to the reference medicine in terms of physicochemical characteristics, adverse events and clinical efficacy. 21
Biosimilars are not considered to be generic copies of innovative products, since traditional generics are considered to be therapeutically and structurally identical to the originator chemical formula with the implication that the active substance is identical to the reference medicine. Most synthetic drugs have a low molecular weight of only a few hundred Daltons (Da). Acetylsalicylic acid has 180 Da with 21 atoms, and omeprazole has 345 Da with 17 carbon atoms. This is not the case for biosimilars since the active principle of a biological is a complex three-dimensional structure with great molecular weight. IFX has 149,000 Da and ADA has 150,000 Da, both have more than 20,000 atoms. Their heterogeneity, dependence on production in cell cultures and highly sophisticated manufacturing processes such as posttranslational modifications through glycosylation, phosphorylation, deamidation, oxygenation and PEGylation make them completely different from single, slightly complex, low-weight molecules and make their production completely different from traditional manufacturing techniques. In addition, they have the ability to induce immune responses (Figure 1). Although the global structure of each biological is known, the process of its manufacture remains unknown since this is part of the intellectual property of the developer. For this reason, current analytical methods cannot characterize these complex molecules with guarantees sufficient for confirming structural bioequivalence with the reference molecules. 22,23,24 Consequently, clinical equivalence can only be proven in clinical studies.
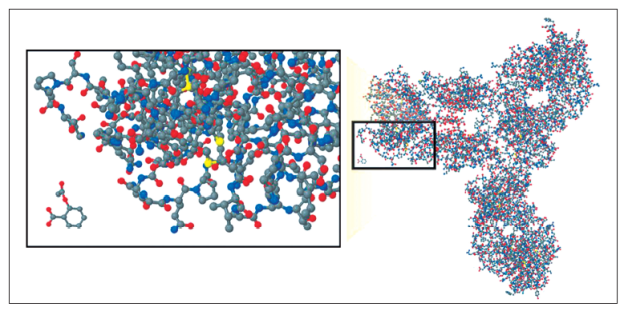
Figure 1 Comparison of a biological monoclonal antibody and an acetylsalicylic acid molecule. Taken from: Kozlowski S, Woodcock J, Midthun K, et al. Developing the nation’s biosimilar program. N Engl J Med. 2011; 365 5: 385-8
When the CT-P13 was compared physicochemically to the originator , their protein structures were found to be identical with the exception of a C-terminal glycine. Higher order structural measurement such as monomeric purity are similar. Comparability of biological activity of the two products was demonstrated through in vitro neutralization of TNF in a similar manner. 12
Studies were performed on healthy volunteers who, according to the FDA, are considered to be the most sensitive for finding differences between biosimilars and reference products. A double-blind 3-arm study of in parallel groups was designed to determine the pharmacokinetic properties of CT-P13 and two IFX formulations, European Remicade® and American Remicade®, after a single infusion of 5 mg/k. The CT-P13 group included 70 healthy subjects, the European Remicade® group included 71 subjects, and the American Remicade® group included 70 subjects. Serum pharmacokinetic levels were measured at before the infusion, immediately after it and at various times until day 57 after infusion. The three formulations were essentially equivalent in terms of maximum concentration (Cmax) of IFX and area under the time curve of serum concentrations. There were no differences in adverse events among the 211 patients evaluated. 25 The position of Health Canada is even more rigorous than that of the FDA, since its policies use supremely pointed and delicate terms such as interchangeability, extrapolation and substitution. Canadian guidelines only considered an agent to be a biosimilar when an approved originator biological already exists. This is also required by the FDA. These guidelines specify the following: 26
A biosimilar can be considered “similar” to the originator after having undergone and passed all “biosimilarity” tests including those for similar biochemical structures, similar pharmacokinetic and pharmacodynamic properties and verification of similarity in clinical studies.
Biosimilars, although they are biological, are not generic biologicals and their authorization does not declare that they are pharmacologically and therapeutically equivalent to the reference biologicals.
A biosimilar should be considered a new biological and should be regulated as such. A biosimilar should not be shielded by the reference biological or extrapolated to include other indications. Nevertheless, Health Canada authorized extrapolation of CT-P13 for all indications for which the originator was approved on October 10, 2016. 27
A biosimilar should not be identified as interchangeable with a biological originator by health professionals.
A biosimilar may not be used as a substitute for a biological originator by a site that dispenses these medicines.
According to a Dutch study, biologicals are responsible for 64% of the total cost of managing CD and 31% of the total cost of managing ulcerative colitis. It is estimated that per milligram cost of biosimilars is between 25% and 40% less than the cost of biologicals. 9 All of this has sparked debate on issues such as safety and concern about bioequivalence regarding the potential for immunogenicity and adverse immunological events.
Three terms in the context the debate about biosimilars are important to understand: extrapolation, substitution and interchangeability. 28
Extrapolation
Extrapolation refers to regulatory agency authorization of a biosimilar that has been approved for treatment of one condition to be used to treat another condition for which it has not been clinically studied. This is precisely what happened with CT-P13. There were extensive in vitro comparative studies, but no clinical studies for treatment of IBD and only two head-to-head comparison in patients with RA and AS. Under the premise that a “great similarity” of studies predicts “highly similar” clinical outcomes (extrapolation), various regulatory agencies including the EMA, FDA, Health Canada and the Colombian agency INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos - National Institute for Drug and Food Surveillance have all authorized CT-P13 for all other indications authorized for the originator biological, Remicade®.
The main argument for accepting extrapolation is that a high degree of analytical “similarity” is the most robust scientific basis for comparing biologicals from independent sources. 9 A more detailed analysis of the two keys studies is helpful. The phase 3 PLANETRA noninferiority study compared CT -P13 and Remicade® in 606 patients with RA. The 95% CI for the therapeutic difference between both molecules for the primary objective was -6% to 10%. This falls within the selected equivalence margin of +/- 15%. The study reported a difference of 2% in effectiveness between CT -P13 and the originator with similar levels of immunogenicity. It should be noted that the patients in this study also received methotrexate which acts as an immunomodulator to prevent or delay formation of anti-infliximab (ATI) antibodies. It therefore prevents differences in immunogenicity between the two drugs being tested from being appreciated early or easily. In addition, the least sensitive disease was chosen as a clinical model for detecting potential differences in efficacy between the biosimilar and the originator. However, under other circumstances these differences might have become evident. In other words, the effectiveness in similarity demonstrated by CT-P13 and Remicade® for RA does not exclude the possibility of clinically significant differences in effectiveness when they are applied in a much more sensitive model such as IBD. 29
Health Canada initially authorized CT-P13 for use in AS and plaque psoriasis using the extrapolation criterion but not authorize its use for treatment of IBD. This has recently been modified, and CT-P13 is now approved for all indications for which Remicade® is approved for treatment of IBD. 27
The phase I PLANETAS study of 250 patients with AD demonstrated bioequivalence between CT-P13 and Remicade® in terms of pharmacokinetics. This methodology cannot be acceptable to regulatory agencies for evaluation of an innovative molecule. Therefore, the concept of real equivalence is questionable. 26
Further investigation is required because efficacy and safety data were extracted from clinical studies of patients with RA, but the mechanism of action of these drugs in relation to RA is basically the neutralization of TNF. However, in relation to IBD these drugs probably act by decreasing the production of cytokines that induce apoptosis of activated monocytes and lymphocytes, 28 promote M2 macrophages and therefore mucosal healing, and result in complementary cell-mediation cytotoxicity. 30 In addition, the clearance rate of IFX is 40% higher in patients with IBD than with AS.
Substitution
Automatic substitution is when one medication is automatically replaced by another medication at the pharmacy without consulting the prescribing doctor.
Interchangeability
According to the FDA, a biosimilar drug can be designated as interchangeable if it is expected to produce the same clinical result as the reference product in a given patient and if repeated alternation between the biosimilar drug and the reference product does not pose safety or efficacy risks. 21,31,32
The EMA does not express recommendations on interchangeability, and substitution guidelines are at the discretion of each European Union (EU) member state. 31,32,33
Exchange for medical reasons is defined as the exchange of one drug for another for clinical reasons related to a patient who responds inadequately to treatment or who presents an intolerable adverse event.
Exchange for non-medical reasons is defined as exchange of one drug for another for a patient receiving appropriate and well tolerated treatment. This situation occurs due to administrative or cost reasons.
The problem of understanding immunological responses in the context of multiple exchanges arises because understanding the response of a given biological medicine is already complicated and not completely understandable. In addition, immunogenic reactions may not be detected until after administration, so it can be difficult to understand and track potential immunogenic reactions after making multiple changes. 33,34,35,36
EMA is supremely strict in the pharmacovigilance of biosimilars since it requires a detailed risk management plan. This plan is considered to be even more important if extrapolation is accepted. 9
An article and commentary on that article have looked at interchangeability since immunogenic comparability between Remicade® and CT-P13 was first investigated. 37,38 Because all monoclonal antibodies generate immunological responses against the drug, the authors wanted to examine if the antibodies produced by Remicade® treatment of patients with IBD also cross-react and recognize CT-P13 to a similar degree. If this is true, one could think of freely interchanging these two drugs in a patient without losing the proven efficacy and safety of the first while reducing cost by as much as 40%. This suggests that, in an individual who has generated high titers of ATI antibodies and who has some adverse reaction or loss of response to the infusion, it is not justified to use the other agent, IFX, since there will be a high probability the patient will present the same adverse event.
In contrast, in an individual who tests negative for ATI antibodies, both agents could be freely exchanged without causing any type of adverse immune reaction because, according to the foregoing, anti-Remicade® antibodies from patients with IBD recognize and functionally inhibit CT-P13 to a similar degree. This would suggest similar immunogenicity for these two IFX agents immunodominant epitopes. Nevertheless, it is important to emphasize that before standardizing this approach for routine use, clinical studies should be conducted in which the cross-reactivity of the serum of patients treated with CT-P13 who have developed both ATI antibodies and anti-CT-P13 antibodies is evaluated. It should be noted that, although there are antibodies shared by Remicade® and CT-P13, there may be other antibodies that are exclusive to each one, as they are similar, but not the same. 37,38
This study demonstrated that anti-adalimumab antibodies (AAA) in patients treated with IBD did not cross-react to CT-P13 or Remicade®, so it inferred that either CT-P13 or Remicade® could replace the other in cases of adverse reactions or lack of response due to AAA and that there would be a good response. Nevertheless, it has also been documented that a patient who has high levels of ATI antibodies may also quickly generate AAA when exposed to the ADA.
Three observational cohort studies of IBD 39,40,41 that evaluated CT-P13 and IFX have reported data on the effectiveness and safety of exchanging a biosimilar for a reference medicine. Criticisms of all of these studies should be noted: all patients were in remission before the reference medicine was replaced with CT-P13, all had small sample numbers, none had control groups of patients who continued to take the reference drug, and the moment of replacement varies greatly among the three studies. Some of the patients had not actually been treated with IFX, and their induction protocol was performed with CT-P13.
In Norway, a prospective observational cohort study was designed to evaluate 78 patients. Forty-six of them had CD, and 28% of those had had previous exposure to biologicals. Thirty two patients had ulcerative colitis sixteen percent of whom had been previously exposed to biologicals. Patients received 5 mg/k of CT-P13 at weeks 0, 2 and 6. At week 14, 79% of patients with ulcerative colitis and 56% of those with CD were found to be in clinical remission after testing negative for C-reactive protein [CRP] and calprotectin. 42
A recently published prospective, observational, multicenter study involving the whole of Hungary has evaluated long-term efficacy and safety. Remission, clinical response and biochemistry were documented at weeks 14, 30 and 54. No patient had received IFX, the originator, in the 12 months prior to the test. Of the 291 patients initially recruited, 184 had CD and 107 had ulcerative colitis. Twenty-five percent of the CD patients and 14% of the UC patients had previously been exposed to anti-TNF, and 60% of CD patients and 52% of UC patients had used concomitant immunosuppressants. After 54 weeks, 100 of these patients could be evaluated. In this study, the percentage of patients with clinical responses to treatment for CD was 83% at 14 weeks, 77% at 30 weeks, 58% at 54 weeks while the percentage of patients in clinical remission was 55% at 14 weeks, 57% at 30 weeks, and 47% at 54 weeks. For patients with ulcerative colitis, clinical response rates were 78% at week 14, 69% at week 30, and 64% at week 54 while clinical remission rates were 59% at week 14, 46% at week 30, and 53% at week 54. Adverse events consisted of 21 (6.6%) incidents of reactions to infusions, 23 (7.9%) infections, and one death. These authors concluded that CT-P13 is safe and effective both for inducing remission and for maintaining it in patients with ulcerative colitis and CD, even though response and remission rates are lower in individuals previously exposed to biologicals. 43
A study of real-life data conducted in Hungary addressed questions of biosecurity. It included 384 consecutive patients, 253 of whom had CD and 131 of whom had ulcerative colitis, who were treated with CT-P13. Of the CD patients, 24.5% had previously received anti-TNF therapy while 14% of the UC patients had previously received anti-TNF therapy. Twenty-one (7.2%) had reactions to infusions during the induction and maintenance phase, and 13 of these had received IFX in the past. Allergic reactions were occurred in 44.4% of these cases while dyspnea and chest pain occurred in 27.7%, blushing in 27.7%, coughing in 22.2%, arthralgia in 11.1% and headaches in 11.1%. CT-P13 should have been suspended and/or replaced with another treatment in 62% (237 patients): it was replaced by adalimumab (ADA) in 146 patients (38.1%) but, despite the above, CT-P13 was continued in 82 patients (21.4%) but with supplementary slow infusion of antihistamines and steroids. The study showed that prior exposure to anti-TNF and having ATI antibodies were predictors of poor prognoses but that of azathioprine (AZA) (borderline) and the normalization of CRP at week 14 were considered to be protective factors. The authors of this study concluded that the use of CT-P13 is safe and the incidence of adverse reactions to infusions was low. 44
The Nor-switch study, also with real-life data, was designed as a randomized, double-blind prospective observational study of 480 patients who had been receiving IFX and who had been clinically stable for more than six months. CT-P13 was compared to IFX for treatment of six immunologically mediated inflammatory diseases (IMID): CD, ulcerative colitis, AD, RA, psoriasis and AP. Half of the patients (240) were switched to CT-P13 and the other 50% (240) continued standard therapy with IFX. The primary objective was to evaluate worsening of the disease after 52 weeks. Overall, 53 of the patients in the IFX group (26.2%) relapsed while 61 patients treated with CT-P13 (29.6%) relapsed. The 95% CI was -12.7% to -3.9%. This indicates that there were no clinically significant differences at the end of the year. Nevertheless, when subgroups were evaluated, it was found that CD patients relapsed more frequently when CT-P13 was used than when IFX was used: 23 patients (36.5%) versus 14 patients (21.2%) respectively with a 95% CI of-29.3 % to -7%) which is clinically significant. 45
It is important to clarify that this study must be analyzed with care since it includes multiple indications and lacks clarity in its definitions, inclusion criteria, exclusion criteria, therapeutic objectives, impact and use of concomitant medication. All of these issues create confusion and prevent this study from having the robust statistical power necessary for rigorous interpretation. 46
The capacity of ulcerative colitis patients for mucosal healing (MH) after induction with CT-P13 was evaluated in 63 patients who had been resistant to conventional treatment or who were steroid-refractory, steroid-dependent or in need of early rescue due to acute toxic colitis. At 14 weeks 82.5% of the patients showed some clinical response, 47.6%) were in steroid-free remission, 47.6% showed steroid-free mucosal healing, and 27% demonstrated complete mucosal healing. In addition, the study determined that trough levels of IFX were correlated with MH. According to these authors, MH is achieved in 2/3 of patients with ulcerative colitis at the end of the induction dose of CT-P13 in week 14. 47
The only study that compared the cost-effectiveness of originator drug with that of its biosimilar in patients with luminal CD was done by the Hungarian group. Their study compared the cost-effectiveness of various biologicals used in Europe and documents costs in nine European countries: Belgium, France, Germany, Hungary, Italy, Holland, Spain, Sweden and England. A probabilistic Markov model was designed to compare the cost-effectiveness of selected treatment sequences with standard management in patients with moderate to severe active luminal CD that did not respond to conventional treatment. A comparison of the use of biosimilars and the originator drug to conventional treatment showed that a biosimilar had the most favorable incremental cost-utility ratios (ICURs) which ranged from € 35,170 in Hungary to € 71,624 in Sweden. A comparison of Remicade® to conventional treatment showed cost increases ranged from € 69,000 in Hungary to € 135,000 in Germany. The inclusion of additional biologicals to the treatment sequences logically increased the costs per year per patient, but was always cheaper when, independently of the chosen sequence (CT-P13-, Remicade®, ADA and/or vedolizumab [VDZ]) at some point the biosimilar was used. Therefore, and according to the authors’ opinion, based on the results of cost-effectiveness, they concluded that CT-P13 can be recommended as a first line treatment for CD that does not respond to conventional treatment. The sequence CT-P13, ADA, VDZ was identified as the most cost-effective and, finally, the use of CT-P13 is a cost-effective alternative to the originator product for the treatment of adults with luminal CD, and this may contribute to increasing access to these products. 48
A Dutch study’s mathematical projection simulating costs 5 years after introduction of biosimilars to the market shows that, at five years, the average saving in total costs per CD patient should be € 9,850, average saving in total costs per UC patient should be € 2250; and total savings in costs for anti-TNF-α therapy in the Netherlands should be € 493 million. 49 The study assumed gradual change to 60% biosimilars, 50% originator and an annual increase in the prescription of anti-TNF-α of 1%.
Finally, I want to concretely describe the positions of the various gastroenterology associations about the introduction of biosimilars as therapeutic options:
The Canadian Gastroenterology Association is against interchangeability of the reference agent and biosimilars and is against automatic substitutions by pharmacies based on cost. 26
The Spanish Society of Digestive Pathology is against extrapolation of the results of the PLANETRA study to the entire population suffering from IBD due to the biological variability of the disease throughout its various states. Instead, it advocates clinical studies of the biosimilar for individual indications, as required for the biological agents of reference. 16
The position of the Italian IBD Group highly recommends that evidence be provided for the efficacy and safety of each biosimilar for treatment of IBD prior to entering the market. They are also against interchangeability of biosimilars and originator drugs and do not support extrapolation through clinical indications, in particular due to the heterogeneity of presentation and clinical course of IBD. 50
In 2013, the European Crohn’s and Colitis Organization (ECCO) emphasized the need for more specific clinical studies of IBD with sufficient statistical power to detect differences not only in the short-term efficacy of biosimilars but also to evaluate long-term safety and immunogenicity. 8 In 2017, following the expert consensus meeting held on October 15, 2016 in Vienna, Austria, 51 a new document was issued. Their position has changed significantly and is much more open. It includes concepts such as:
Clinical equivalence studies for the most sensitive prescription indications can provide the basis for extrapolation. Therefore, information on the use of biosimilars for another sensitive indications can be used for IBD treatment.
Only observational studies with long-term registries of series from real-life settings can demonstrate the safety of these medications.
Adverse events and loss of response due to the immunogenicity of a biological originator are not expected to be avoided through the use of their biosimilar.
Switching from the originator to the biosimilar is accepted, although there is still a lack of clinical evidence regarding reverse direction switches, multiple interchangeability and interchangeability between and among biosimilars.
Interchanges between an originator and a biosimilar can be done once there is broad knowledge and clarity regarding this among all the actors involved including prescribing physicians, pharmacists, nurses, patients and payers. A recent survey of 1,181 patients by the European Federation of Crohn’s and Colitis Associations found that only 38% of respondents knew about biosimilars. More importantly, it found that 55.9% of those who answered think that the lower cost of these drugs should not take precedence over safety and therapeutic efficacy.
The position of the Crohn and Colitis Foundation of America (CCFA) on biosimilars emphasizes the need to make a shared and transparent decision against automatic substitutions and allow providers to determine whether replacement of a reference product by a biosimilar is acceptable. In addition, precautions should be taken against interchangeability until there is further evidence of safety, efficacy, and immunogenicity. 52
The British Society of Gastroenterology has published guidelines which support initiation of therapy with CT-P13. In addition, they have approved replacing originator IFX in stable patients with a biosimilar, but they do not recommend automatic substitution in pharmacies without the knowledge of the attending physician. 53
All societies recommend separate and unique identification numbers and names for biosimilars in accordance with non-patented international names as required by the World Health Organization (WHO) to prevent confusion among patients, providers and pharmacies regarding originator drugs and biosimilars. In 2014, the WHO expert group on international non-proprietary names (INN) proposed a 2-component system for identification of biosimilars. The first component of the name would be the INN, and the second part would be a unique code (biological identifier) consisting of four randomly assigned letters. 54
In Europe, a biosimilar shares the same INN as its originator. This decision was made in October 2013 with the aim of maintaining a close relationship with the originator since assignment of a different INN could create distrust of these products in the public and among health care personnel. 55
In January 2017, the FDA published guidelines for naming non-patented biosimilar agents that aim to avoid confusion and inadvertent assumptions of interchangeability. Names will include four letters suffixes without meaning. According to this rule, Inflectra® will add the suffix “dyyb” to differentiate it from infliximab. In other words it will become infliximab-dyyb. Importantly, this rule also affects agents that had already been approved such as Remicade® whose official name will become infliximab-hjmt. This avoids imprecise perceptions that a biosimilar has clinically significant differences or is inferior for the prescribed indications. 31,32
Conclusion
If bioequivalence and interchangeability can be demonstrated for biosimilars and the originator molecule for patients with IBD, based on cost savings this would open a new door for faster approval of these drugs for patients who require them as well as for combinations of them with other molecules (VDZ). The latter is not done currently because of the high costs, 28 but the central dilemmas for patients, prescribers and insurers are to what extent a biosimilar is interchangeable with its originator, under which circumstances are they interchangeable, and for which patients are they interchangeable. To complicate matters further, the arrival of new biosimilars for any originator raises the questions of whether or not they can also be exchanged freely for the originator and whether or not they can be exchanged freely among themselves with the same level of safety and efficacy (Figures 2 and 3).
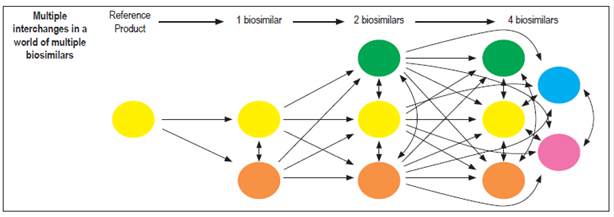
Figure 2 Diagram of multiple exchanges among biosimilars. Taken from: Faccin F et al. Expert Opin Biol Ther. 2016; 16 (12): 1445-53.
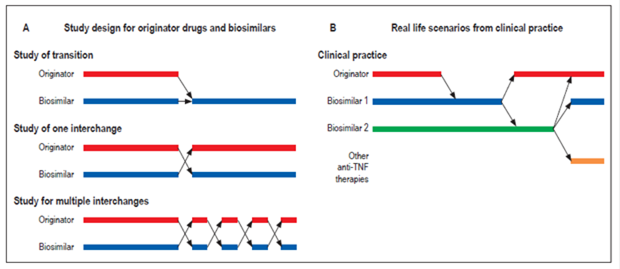
Figure 3 Schematic real-life scenarios of multiple exchanges between biosimilars and references. Taken from: Faccin F et al. Expert Opin Biol Ther. 2016; 16 (12): 1445-53.
At a round table on biosimilars in Brussels on January 12, 2016, European regulators and medical societies concluded that prescribers and regulators had different understandings of the concept of biosimilars, even though this situation is changing as more information about these products becomes known. Above all real life experience in countries where administration of biosimilars has increased, and where interchangeability has also increased, has been positive. Therefore, rather than seeing biosimilars as threats, they should be approached as opportunities. This should promote multidirectional communication among all actors and in the process help define interchangeability, pharmacovigilance, traceability and other issues. 56
Ultimately, biosimilars are here to stay. This is a reality that must be lived with even though existing evidence only supports biosimilarity and does not support interchangeability which still has no evidence. For this reason the FDA is working on draft guidelines about the topic. The data, though still not conclusive, suggests that a single switch or change under medical supervision may be effective, but this is different from interchangeability. The main argument for production and sales of these new anti-TNFs for management of immune-mediated inflammatory diseases (IMID) has been cost savings of up to 40%. In reality, since 2013 when Remicade® was first marketed in the EU, the discount has been between 10% and 30%, with an average of 25%. Probably, in our country it is even lower. 12 The knowledge necessary for using these drugs optimally is the best tool available to regulators, paying agents and patients who, after all, are our reason for being.
Acknowledgments
We would like to thank Dr. Harold Perdomo, immunologist (Medical Scientific Liaison, Abbvie, Bogotá, Colombia) and Dr. Natali Serra-Bonett (Regional Lead Biotherapeutics LATAM Global Medical Affairs, Abbvie, Mexico City, Mexico).
REFERENCES
1. European Medicines Agency. Assessment report: Remsima (infliximab). European Medicines Agency [internet] 2013 [acceso el 12 de febrero de 2017]. Disponible en: Disponible en: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf . [ Links ]
2. Rickwood S, Iervolino A. Shaping the biosimilars opportunity: a global perspective on the evolving biosimilars landscape. IMS Health [internet] 2011 [acceso el 9 de junio de 2017]. Disponible en: Disponible en: https://weinberggroup.com/pdfs/Shaping_the_biosimiliars_opportunity_A_global_perspective_on_the_evolving_biosimiliars_landscape.pdf. [ Links ]
3. Miller KL, Lanthier M. Regulatory watch: innovation in biologic new molecular entities: 1986-2014. Nat Rev Drug Discov. 2015;14(2):83. https://doi.org/10.1038/nrd4535 [ Links ]
4. Gaviria A, Vaca González CP, Muñoz CG, et al. El debate de la regulación de medicamentos biotecnológicos: Colombia en el contexto mundial. Rev Panam Salud Publica. 2016;40(1):40-7. [ Links ]
5. Aitken M. Delivering on the potential of biosimilar medicines: The role of functioning competitive markets. IMS Health [internet] 2016 [acceso el 9 de junio de 2017]. Disponible en: Disponible en: http://www.imshealth.com/files/web/IMSH%20Institute/Healthcare%20Briefs/DocDocume/IMS_Institute_Biosimilar_Brief_March_2016.pdf . [ Links ]
6. von Schaper E. Celltrion’s infliximab copy shows path to biosimilars in US. Nat Biotechnol. 2016;34(5):454-5. https://doi.org/10.1038/nbt0516-454 [ Links ]
7. Pavlovic M, Girardin E, Kapetanovic L, et al. Similar biological medicinal products containing recombinant human growth hormone: European regulation. Horm Res. 2008;69(1):14-21. [ Links ]
8. Danese S, Gomollón F. Governing Board and Operational Board of ECCO. ECCO position statement: the use of biosimilar medicines in the treatment of inflammatory bowel disease (IBD). J Crohns Colitis. 2013;7(7):586-9. https://doi.org/10.1016/j.crohns.2013.03.011 [ Links ]
9. Gomollón F. Biosimilars in inflammatory bowel disease: ready for prime time? Curr Opin Gastroenterol. 2015;31(4):290-5. https://doi.org/10.1097/MOG.0000000000000184 [ Links ]
10. Danese S, Bonovas S, Peyrin-Biroulet L. Biosimilars in IBD: from theory to practice. Nat Rev Gastroenterol Hepatol. 2017;14(1):22-31. https://doi.org/10.1038/nrgastro.2016.155 [ Links ]
11. Reinisch W, Louis E, Danese S. The scientific and regulatory rationale for indication extrapolation: a case study based on the infliximab biosimilar CT-P13. Expert Rev Gastroenterol Hepatol. 2015;9 Suppl 1:17-26. https://doi.org/10.1586/17474124.2015.1091306 [ Links ]
12. Ha CY, Kornbluth A. A critical review of biosimilars in IBD: the confluence of biologic drug development, regulatory requirements, clinical outcomes, and big business. Inflamm Bowel Dis. 2016;22(10):2513-26. https://doi.org/10.1097/MIB.0000000000000886 [ Links ]
13. Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72(10):1605-12. https://doi.org/10.1136/annrheumdis-2013-eular.1548 https://doi.org/10.1136/annrheumdis-2012-203091 [ Links ]
14. Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis . 2013;72(10):1613-20. https://doi.org/10.1136/annrheumdis-2012-203090 [ Links ]
15. Requisitos y el procedimiento para las evaluaciones farmacológica y farmacéutica de los medicamentos biológicos en el trámite del registro sanitario. Ministerio de Salud y Protección Social, decreto número 1782 del 18 de septiembre de 2014. [ Links ]
16. Argüelles-Arias F, Barreiro-de-Acosta M, Carballo F, et al. Joint position statement by “Sociedad Española de Patología Digestiva” (Spanish Society of Gastroenterology) and “Sociedad Española de Farmacología” (Spanish Society of Pharmacology) on biosimilar therapy for inflammatory bowel disease. Rev Esp Enferm Dig. 2013;105(1):37-43. https://doi.org/10.4321/S1130-01082013000100006 [ Links ]
17. Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337(15):1029-35. https://doi.org/10.1056/NEJM199710093371502 [ Links ]
18. Rutgeerts P, Vermeire S, Van Assche G. Biological therapies for inflammatory bowel diseases. Gastroenterology. 2009;136(4):1182-97. https://doi.org/10.1053/j.gastro.2009.02.001 [ Links ]
19. Pache I, Rogler G, Felley C. TNF-alpha blockers in inflammatory bowel diseases: practical consensus recommendations and a user’s guide. Swiss Med Wkly. 2009;139(19-20):278-87. [ Links ]
20. Ford AC, Sandborn WJ, Khan KJ, et al. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):644-59. https://doi.org/10.1038/ajg.2011.73 https://doi.org/10.1038/ajg.2011.71 https://doi.org/10.1038/ajg.2011.70 [ Links ]
21. Chingcuanco F, Segal JB, Kim SC, et al. Bioequivalence of biosimilar tumor necrosis factor-α inhibitors compared with their reference biologics: a systematic review. Ann Intern Med. 2016;165(8):565-74. https://doi.org/10.7326/M16-0428 [ Links ]
22. Schellekens H. Biosimilar therapeutics-what do we need to consider? NDT Plus. 2009;2(Suppl_1):i27-36. [ Links ]
23. Roger SD. Biosimilars: how similar or dissimilar are they? Nephrology (Carlton). 2006;11(4):341-6. https://doi.org/10.1111/j.1440-1797.2006.00594.x [ Links ]
24. Kozuch PL, Hanauer SB. General principles and pharmacology of biologics in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35(4):757-73. [ Links ]
25. Park W, Lee SJ, Yun J, et al. Comparison of the pharmacokinetics and safety of three formulations of infliximab (CT-P13, EU-approved reference infliximab and the US-licensed reference infliximab) in healthy subjects: a randomized, double-blind, three-arm, parallel-group, single-dose, Phase I study. Expert Rev Clin Immunol. 2015;11 Suppl 1:S25-31. https://doi.org/10.1586/1744666X.2015.1090311 [ Links ]
26. Devlin SM, Bressler B, Bernstein CN, et al. Overview of subsequent entry biologics for the management of inflammatory bowel disease and Canadian Association of Gastroenterology position statement on subsequent entry biologics. Can J Gastroenterol. 2013;27(10):567-71. https://doi.org/10.1155/2013/327120 [ Links ]
27. Health Canada. Regulatory decision summary inflectra supplemental new drug submission 2016/06/10. 2016 [acceso el 12 febrero de 2017]. Disponible en: Disponible en: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/health-canada-new-drug-authorizations-2016-highlights.html [ Links ]
28. Vande Casteele N, Sandborn WJ. IBD: Indication extrapolation for anti-TNF biosimilars. Nat Rev Gastroenterol Hepatol . 2015;12(7):373-4. [ Links ]
29. Feagan BG, Choquette D, Ghosh S, Gladman DD, Ho V, Meibohm B, Zou G, Xu Z, Shankar G, Sealey DC, Russell AS. The challenge of indication extrapolation for infliximab biosimilars. Biologicals. 2014 Jul;42(4):177-83. https://doi.org/10.1016/j.biologicals.2014.05.005 [ Links ]
30. Levin AD, Wildenberg ME, van den Brink GR. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J Crohns Colitis . 2016;10(8):989-97. https://doi.org/10.1093/ecco-jcc/jjw053 [ Links ]
31. Food and Drug Administration. Nonpropietary naming of biological products. FDA [internet] 2017 [acceso el 12 febrero de 2017]. Disponible en: Disponible en: https://www.fda.gov/downloads/drugs/guidances/ucm459987.pdf . [ Links ]
32. Food and Drug Administration. Designation of official names and proper names for certain biological products: proposed rule. FDA [internet] 2015 [acceso el 12 febrero de 2017]. Disponible en: Disponible en: https://www.gpo.gov/fdsys/pkg/FR-2015-08-28/pdf/2015-21382.pdf . [ Links ]
33. European Commission. Consensus Information Paper 2013. What you need to know about Biosimilar Medicinal Products. European Commission [internet] 2013 [acceso el 12 febrero de 2017]. Disponible en: Disponible en: http://www.medicinesforeurope.com/wp-content/uploads/2016/03/biosimilars_report_en.pdf . [ Links ]
34. Vermeer NS, Spierings I, Mantel-Teeuwisse AK, et al. Traceability of biologicals: present challenges in pharmacovigilance. Expert Opin Drug Saf. 2015;14(1):63-72. https://doi.org/10.1517/14740338.2015.972362 [ Links ]
35. Dörner T, Strand V, Castañeda-Hernández G, et al. The role of biosimilars in the treatment of rheumatic diseases. Ann Rheum Dis . 2013;72(3):322-8. https://doi.org/10.1136/annrheumdis-2012-202715 [ Links ]
36. Casadevall N, Nataf J, Viron B, et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med . 2002;346(7):469-75. https://doi.org/10.1056/NEJMoa011931 [ Links ]
37. Ben-Horin S, Yavzori M, Benhar I, et al. Cross-immunogenicity: antibodies to infliximab in Remicade-treated patients with IBD similarly recognise the biosimilar Remsima. Gut. 2016;65(7):1132-8. [ Links ]
38. Márquez Velásquez JR. Inmunogenicidad cruzada: anticuerpos para infliximab en pacientes con enfermedad inflamatoria intestinal tratados con Remicade reconocen similarmente al biosimilar Remsima. Enfermedad Inflamatoria Intestinal al Día. 2016;15:19-21. https://doi.org/10.1016/j.eii.2015.12.002 [ Links ]
39. Kang YS, Moon HH, Lee SE, et al. Clinical experience of the use of CT-P13, a biosimilar to infliximab in patients with inflammatory bowel disease: A Case Series. Dig Dis Sci. 2015;60(4):951-6. https://doi.org/10.1007/s10620-014-3392-z [ Links ]
40. Jung YS, Park DI, Kim YH, et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: A retrospective multicenter study. J Gastroenterol Hepatol. 2015;30(12):1705-12. https://doi.org/10.1111/jgh.12997 [ Links ]
41. Hlavaty T, Krajcovicova A, Sturdik I, et al. Biosimilar infliximab CT-P13 treatment in patients with inflammatory bowel disease - a one-year, single centre retrospective study. Journal of Gastroenterology and Hepatology. 2016;70(1):27-36. https://doi.org/10.14735/amgh201627 [ Links ]
42. Jahnsen J, Detlie TE, Vatn S, et al. Biosimilar infliximab (CT-P13) in the treatment of inflammatory bowel disease: A Norwegian observational study. Expert Rev Gastroenterol Hepatol . 2015;9 Suppl 1:45-52. https://doi.org/10.1586/17474124.2015.1091308 [ Links ]
43. Gecse KB, Lovász BD, Farkas K, et al. Efficacy and safety of the biosimilar infliximab ct-p13 treatment in inflammatory bowel diseases: a prospective, multicentre, nationwide cohort. J Crohns Colitis . 2016;10(2):133-40. [ Links ]
44. Bálint A, Rutka M, Végh Z, et al. Frequency and characteristics of infusion reactions during biosimilar infliximab treatment in inflammatory bowel diseases: results from Central European nationwide cohort. Expert Opin Drug Saf . 2017;16(8):885-890. [ Links ]
45. Jørgensen K et al. (2016) ‘LB15 - Biosimilar infliximab (CT-P13) is not inferior to originator infliximab: results from the 52-week randomized NOR-SWITCH trial.’ Abstract presented at the United European Gastroenterology (UEG) Week meeting 2016;15-19. [ Links ]
46. Faccin F, Tebbey P, Alexander E, Wang X, Cui L, Albuquerque T. The design of clinical trials to support the switching and alternation of biosimilars. Expert Opin Biol Ther. 2016;16(12):1445-53. [ Links ]
47. Farkas K, Rutka M, Golovics PA, et al. Efficacy of Infliximab Biosimilar CT-P13 Induction Therapy on Mucosal Healing in Ulcerative Colitis. J Crohns Colitis . 2016;10(11):1273-1278. [ Links ]
48. Rencz F, Brodszky V, Baji P, et al. Cost-utility of biosimilar infliximab (Inflectra©) for the treatment of luminal crohn´s disease in nine European countries. United European Gastroenterology Journal. 2016;2 (Supplement 1). [ Links ]
49. Severs M, Oldenburg B, van Bodegraven AA, et al. The economic impact of the introduction of biosimilars in inflammatory bowel disease. J Crohns Colitis . 2017;11(3):289-96. [ Links ]
50. Annese V, Vecchi M. Italian Group for the Study of IBD (IG-IBD). Use of biosimilars in inflammatory bowel disease: statements of the italian group for inflammatory bowel disease. Dig Liver Dis. 2014;46(11):963-8. https://doi.org/10.1016/j.dld.2014.07.019 [ Links ]
51. Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J Crohns Colitis . 2017;11(1):26-34. https://doi.org/10.1093/ecco-jcc/jjw198 [ Links ]
52. Crohn’s and Colitis Foundation of America Position Statement on Biosimilars. Febrero 9 de 2016 [acceso el 11 de diciembre de 2017]. Disponible en: Disponible en: http://www.crohnscolitisfoundation.org/assets/pdfs/advocacy/ccfa-comments-to-fda-re.pdf . [ Links ]
53. British Society of Gastroenterology. BSG Guidance on the use of biosimilar infliximab CT-P13 in inflammatory bowel disease. BSG [internet] 2016 [acceso el 22 de enero de 2017]. Disponible en: Disponible en: http://www.bsg.org.uk/images/stories/docs/clinical/guidance/bsg_infliximab_guidagui_16.pdf . [ Links ]
54. World Health Organization. Biological qualifier: an INN proposal. WHO [internet] 2014 [acceso el 12 febrero de 2017]. Disponible en: Disponible en: http://www.who.int/medicines/services/inn/bq_innproposal201407.pdf . [ Links ]
55. European Commission , Health and Consumers Directorate General [acceso el 11 de diciembre de 2017]. Disponible en: Disponible en: www.ema.europa.eu/docs/en_GB/document_library/Report/2017/12/WC500239794.pdf [ Links ]
56. Annese V, Avendaño-Solá C, Breedveld F, et al. Roundtable on biosimilars with European regulators and medical societies, Brussels, Belgium, 12 January 2016. GaBI Journal. 2016;5(2):74-83. https://doi.org/10.5639/gabij.2016.0502.019 [ Links ]
Received: March 15, 2017; Accepted: October 06, 2017











 texto en
texto en 





