Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Boletín de Investigaciones Marinas y Costeras - INVEMAR
versión impresa ISSN 0122-9761
Bol. Invest. Mar. Cost. vol.48 no.1 Santa Marta ene./jun. 2019 Epub 30-Ago-2019
https://doi.org/10.25268/bimc.invemar.2019.48.1.756
Research Articles
Fishes associating with shallow water echinoids at Roatán, Honduras
1 Department of Biology, Pacific Union College, 1 Angwin Ave., Angwin, CA 94508, USA
We studied the association of 11 species of fishes with 5 species of echinoids at Roatán, Honduras, from 27 August to 1 September 2017. Fishes associated most frequently with the echinoid Diadema antillarum (34.3% of echinoids, six fish species, n = 146 echinoids), followed by Echinometra viridis (25.0%, three fish species, n = 12), Echinometra lucunter (7.5%, ten fish species, n = 1,834), Eucidaris tribuloides (3.4%, four fish species, n = 116), and Tripneustes ventricosus (7.1%, one fish species, n = 28). Of 196 fishes seeking shelter beside echinoids, Malacoctenus aurolineatus was the most common (41.8% of fishes, three echinoid species), followed by Stegastes adustus (38.8%, three echinoid species), Stegastes diencaeus (6.6%, three echinoid species), Sargocentron coruscum (6.1%, five echinoid species), Chaetodon capistratus (1.5%, one echinoid species), Gobioclinus filamentosus (1.5%, one echinoid species), Pomacanthus paru (1.0%, two echinoid species), Labrisomus nuchipinnis (1.0%, two echinoid species), Equetus punctatus (0.5%, one echinoid species), Microspathodon chryurus (0.5%, one echinoid species), and Thalassoma bifasciatum (0.5%, one echinoid species). None of the fishes associated exclusively with echinoids or was specialized for associating with echinoids, indicating the association was facultative. All fishes were small (< 12 cm). Fishes associated most frequently with the longest-spined echinoid, D. antillarum, supporting the hypothesis that fishes seek shelter among the spines of echinoids to benefit from increased protection from predation.
KEYWORDS: Caribbean Sea; Coral reefs; Ectosymbionts; Facultative association
Se realizó el estudio de la asociación de 11 especies de peces con cinco especies de equinoideos en Roatán, Honduras, del 27 de agosto al 1 de septiembre de 2017. Los peces se asociaron con mayor frecuencia con el equinoideo Diadema antillarum (34,3% de los equinoideos, seis especies de peces, n = 146 equinoideos), seguido por Echinometra viridis (25,0%, tres especies de peces, n = 12), Echinometra lucunter (7,5%, diez especies de peces, n = 1.834), Eucidaris tribuloides (3,4%, cuatro especies de peces, n = 116), y Tripneustes ventricosus (7,1%, una especie de pez, n = 28). De los 196 peces que buscaban refugio al lado de los equinoideos, Malacoctenus aurolineatus fue el más común (41,8% de los peces, tres especies de equinoideos), seguido de Stegastes adustus (38,8%, tres especies de equinoideos), Stegastes diencaeus (6,6%, tres erizos equinoideos), Sargocentron coruscum (6,1%, cinco especies de equinoideos), Chaetodon capistratus (1,5%, una especie de equinoideo), Gobioclinus filamentosus (1,5%, una especie de equinoideo), Pomacanthus paru (1,0%, dos especies de equinoideos), Labrisomus nuchipinnis (1,0%, dos especies de equinoideos), Equetus punctatus (0,5%, una especie de equinoideo), Microspathodon chryurus (0,5%, una especie de equinoideo), y Thalassoma bifasciatum (0,5%, una especie de equinoideo). Ninguno de los peces estaba asociado exclusivamente con equinoideos o estaba especializado para asociarse con equinoideos, lo que indica que la asociación era facultativa. Todos los peces eran pequeños (< 12 cm). Los peces se asociaron con mayor frecuencia con el equinoideo de espinas más largas, D. antillarum, apoyando la hipótesis de que los peces buscan refugio entre las espinas de los equinoideos para beneficiarse de una mayor protección contra la depredación.
PALABRAS CLAVE: Mar Caribe; Arrecifes de coral; Ectosimbiontes; Asociación facultativa
INTRODUCTION
Echinoids (sea urchins) comprise an important component of shallow coral reef ecosystems, providing nutrients, microhabitats, and shelter for a variety of ectosymbiotic organisms (Clark, 1976; Birkeland, 1989; Carpenter, 1997; Glynn and Enochs, 2011; Goldberg, 2013; Steneck, 2013), including fishes (Karplus, 2014). In the tropical western Atlantic Ocean a variety of fishes have been reported associating with echinoids (Teytaud, 1971; Helfman et al, 1982; Schoppe, 1991; Schoppe and Werding, 1996; Monroy López and Solano, 2006; Townsend and Bologna, 2007; Giglio et al., 2017), yet much remains to be learned about the fishes associating with echinoids and their ecological relationships.
Several studies provide evidence that ectosymbionts associate with the spines of echinoids, especially the longer-spined species, to gain protection from predators. For example, several fishes (Magnus, 1967; Tamura, 1982; Gould et al., 2014; Giglio et al., 2017) and crustaceans (Castro, 1974; Joseph et al., 1998; Hayes et al., 2016) prefer echinoid individuals, species, or models with longer or denser spines. Several fishes (Lachner, 1955; Eibl-Eibesfeldt, 1961; Strasburg, 1966; Magnus, 1967; Fricke, 1970; Tamura, 1982; Hartney and Grorud, 2002; Gould et al., 2014) and crustaceans (Lewis, 1956; Chace, 1969; Fricke and Hentschel, 1971; Bruce, 1976, 1982; Patton et al., 1985) associate almost exclusively with long-spined echinoid species, retreating deeper into their spines when threatened; some of these species match the color of echinoid hosts, possess dark horizontal lines on their bodies which are aligned with echinoid spines, or change color when departing from echinoid hosts to forage away from them.
In this study we provide data on the frequency of 11 species of fishes seeking shelter among five species of echinoids on the island of Roatán, Honduras, in the western Caribbean Sea. We further test the hypothesis that fishes associate most frequently with the longest-spined species of echinoid, which is consistent with the hypothesis that ectosymbionts seek shelter among the spines of echinoids to benefit from increased protection from predation.
STUDY AREA
Roatán is one of the Bay Islands of Honduras, which are located on the Bonacca Ridge, a crest on the south edge of the deep Cayman (or Bartlett) Trough, in the western Caribbean Sea. The island is 47 km long and 4 km wide, and is located 47 km north of the Honduran mainland, between 16°25.9' and 16°16.0' N, and between 86°36.2' and 86°11.4' W. Roatán is formed of mostly igneous and metamorphic rocks. Limestone outcrops, representing uplifted fossilized reefs, occur along the shore, alternating with sandy beaches and mangroves. A mosaic of fringing and barrier reefs surround the island, with patch reefs in shallow lagoons. The limestone rocks of the intertidal and subtidal zones in our study sites at West Bay and West End were extremely rough, with an abundance of natural crevices varying greatly in size, although most (if not all) crevices appear to have been enhanced by the rock-boring activities of echinoids, which are well known for boring into rocks (Bak, 1994). The island's geology is described by McBirney and Bass (1967) and Lallemant and Gordon (1999), and its marine ecosystems are described by Wells (1988) and Keck (2000).
METHODS
Sampling methods
From 27 August to 1 September 2017, we surveyed the association of fishes with echinoids in crevices along the rocky shoreline at West Bay and West End in Roatán. Snorkeling equipment was used to survey echinoids along the rocky shoreline in water < 2 m deep within 3 m of rocky shores. To avoid sampling the same echinoids twice, the study sites were divided into sections and each section was surveyed by a group of two, with one surveying echinoids and the other recording data, along approximately 1,000 m of coast within an area of approximately 3,000 m2 (measured with Google Earth; www.google.com/earth). Surveys were conducted only during periods of good weather, when skies were clear or partly cloudy and the sea surface was relatively smooth.
We carefully inspected each echinoid for fishes. An association was considered to occur when a fish sought shelter within 2.5 cm of the spines of the echinoid and remained within 5 cm of the spines for at least 10 sec when disturbed. Because many echinoids were partially hidden within a rock or coral crevice, some small fishes may have been overlooked, although we did not inspect echinoids that were mostly hidden within a crevice. Each echinoid and fish species was identified in the field or photographed and subsequently identified based on the field guides of Humann and DeLoach (2002a, 2002b) and Charteris (2012). The number of individuals of each fish species associating with each echinoid species was recorded on underwater writing slates. For a subset of fishes, we counted the number of crevices occupied by echinoids that each fish visited during a 10 min period. Although we did not attempt to measure the density of echinoids, the samples sizes provide a general estimate of the relative abundance of each species.
Statistical analysis
The percent frequency of echinoid hosts occupied by fishes and the mean number of fishes per echinoid were calculated for each fish species and for all fishes combined. Chi-square analyses of contingency tables (χ2 statistic; Zar, 2010) were calculated to compare the proportions of fishes associating with different species of echinoids, thereby testing the prediction that fishes associated most frequently with the longest-spined species of echinoid. To avoid an expected frequency of < 1 in our chi-square tests, we excluded data for species of echinoids in which no fish was observed. When sample sizes were too small to avoid an expected frequency of < 1, no chi-square test was used.
Taxonomy
The taxonomy of echinoids is based on Hendler et al. (1995) and the taxonomy of fishes is based on Froese and Pauly (2019).
RESULTS
Differences in association among echinoids
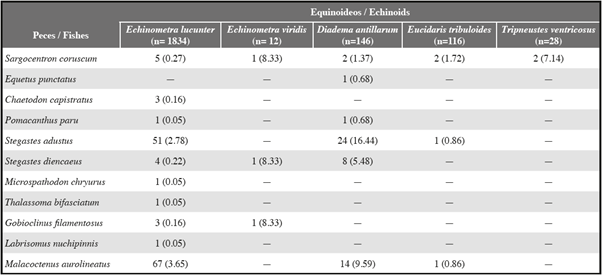
We identified six species of echinoids in crevices along the rocky shorelines, of which five species hosted at least one species of fish. The frequency of fishes seeking shelter under the spines of echinoids (Table 1) differed significantly among the five species of echinoids (χ2 = 124.83, df 4, P < 0.001) and was greatest for the longest-spined species, the diadematid echinoid Diadema antillarum, which accounted for 88.2% of the overall χ2 value. Six fish species associated with 34.3% of D. antillarum, with an average of 0.34 fish per echinoid (n = 146). Three fish species associated with 25.0% of the echinometrid echinoid Echinometra viridis, with an average of 0.25 fish per echinoid (n = 12). Ten fish species associated with 7.5% of the echinometrid echinoid Echinometra lucunter, with an average of 0.07 fish per echinoid (n = 1,834). Four fish species associated with 3.4% of the cidarid echinoid Eucidaris tribuloides, with an average of 0.03 fish per echinoid (n = 116). One fish species associated with 7.1% of the toxopneustid echinoid Tripneustes ventricosus, with an average of 0.07 fish per echinoid (n = 28). No fish species associated with the toxopneustid echinoid Lytechinus variegatus, of which only one was observed. During our initial observations we never observed more than one fish with an echinoid, but a few times we observed a fish subsequently joining another fish with an echinoid.
Differences in association among fishes
We observed 196 fishes of 11 species seeking shelter beside echinoids (Table 1). The fish species differed significantly in their frequency of association with echinoids (χ2 = 527.54, df = 10, P < 0.001). All fishes associating with echinoids were small, with a total length < 12 cm, and most were < 6 cm.
The labrisomid blenny Malacoctenus aurolineatus was the most common associate of echinoids, comprising 41.8% of fishes associating with echinoids. It associated with three echinoid species but associated more frequently with the longest-spined species, D. antillarum, than with the other two species (χ2 = 15.71, df = 2, P < 0.001; Table 1). Both juveniles and adults dwelled within crevices occupied by echinoids, rested on the substrate within crevices, usually sought shelter beside echinoids, and were rarely seen > 1 m from an echinoid (Fig. 1D). Two other labrisomid blennies, Gobioclinus filamentosus and Labrisomus nuchipinnis, behaved similarly but associated much less frequently with echinoids, accounting for 1.5% and 1.0%, respectively, of fishes associating with echinoids. Adults of G. filamentosus (no juveniles observed) associated only with E. lucunter and juveniles of L. nuchipinnis (no adults observed) associated with both E. lucunter and E. viridis (Table 1). None of these fishes were observed > 1 m from an echinoid.
The pomacentrid damselfish Stegastes adustus was the second most common associate of echinoids, comprising 38.8% of the fishes seeking shelter beside echinoids. It associated with three echinoid species but associated more frequently with the longest-spined species, D. antillarum, than with the other two species (χ2 = 74.87, df = 2, P < 0.001; Table 1). Two other pomacentrid damselfishes, Stegastes diencaeus and Microspathodon chryurus, sought shelter beside echinoids, accounting for 6.6% and 0.5%, respectively, of fishes associating with echinoids. Stegastes diencaeus associated with three echinoid species and M. chryurus associated only with E. lucunter (Table 1). Although juveniles and adults of all three pomacentrid species frequently swam into crevices when disturbed, only juveniles sought shelter beside echinoids (Fig. 1C). Both juveniles and adults of all three species were frequently seen > 1 m from echinoids.

Photography: A. by F. E. Hayes; B.-D. by S. T. Richards.
Figure 1 Examples of fishes associating with echinoids at Roatán, Honduras: (A) juvenile Sargocentron coruscum with Echinometra viridis (left) and E. lucunter (right); (B) juvenile Equetus punctatus with Diadema antillarum; (C) juvenile Stegastes diencaeus with D. antillarum; and (D) adult Malacoctenus aurolineatus with D. antillarum.
The holocentrid squirrelfish Sargocentron coruscum comprised 6.1% of fishes seeking shelter beside echinoids. It was observed associating with all five echinoid species (Table 1). Only juveniles sought shelter beside echinoids (Fig 1A). Both juveniles and adults were often observed > 1 m from echinoids.
The four remaining species collectively comprised only 3.6% of fishes seeking shelter beside echinoids. Small juveniles of the chaetodontid butterflyfish Chaetodon capistratus, comprising 1.5% of fishes, sought shelter beside E. lucunter (Table 1). Small juveniles of the pomacanthid angelfish Pomacanthus paru, accounting for 1.0% of fishes, associated with D. antillarum and E. lucunter (Table 1). A tiny juvenile of the sciaenid drum Equetus punctatus, representing 0.5% of fishes associating with echinoids, swam back and forth repeatedly between two large D. antillarum approximately 15 cm apart in a large crevice (Fig. 1B; Table 1). Although juveniles of the labrid wrasse Thalassoma bifasciatum frequently swam into crevices when disturbed, they quickly departed; however, a tiny juvenile (c. 2.5 cm) once lingered beside an E. lucunter (Table 1) and repeatedly sought shelter with other nearby E. lucunter (Table 2). Juveniles and adults of C. capistratus, P. paru, and T. bifasciatum were often observed > 1 m from echinoids. The only other E. punctatus observed was > 1 m from an echinoid.
Table 2 Number of different crevices occupied by echinoids that were entered by an individual fish during a 10-min period.
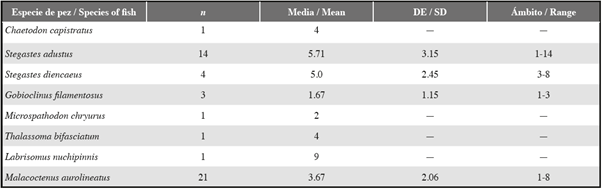
Most individuals of eight species of fishes ventured repeatedly outside of crevices inhabited by echinoids and retreated repeatedly into nearby crevices inhabited by echinoids during 10-min observation periods (Table 2). Only a small proportion of individuals of S. adustus, G. filamentosus, and M. aurolineatus lingered beside a single echinoid during a full 10-min observation period (Table 2).
DISCUSSION
Previous studies revealed that the gobiesocid clingfish Acyrtus rubiginosus is an obligate inquilinistic commensal in burrows of E. lucunter at nearby Colombia (Schoppe, 1991; Schoppe and Werding, 1996; Monroy López and Solano, 2006). Acyrtus rubiginosus has been reported associating with D. antillarum in the Bahamas (Briggs, 1955) and with both D. antillarum and E. lucunter at St. Croix, US Virgin Islands (Teytaud, 1971). At the latter locality the gobiid goby Ginsburgellus novemlineatus was also reported associating with both D. antillarum and E. lucunter (Teytaud, 1971). Both of these species are very small (max lengths 3.5 cm and 2.5 cm, respectively; Froese and Pauly, 2019) and easily fit within the burrows of echinoids. However, we did not encounter either of these fish species in Roatán, where they apparently have not been recorded (Charteris, 2012), although A. rubiginosus has been reported from nearby Utila and Cayos Cochinos (Robertson and Van Tassell, 2015).
At Roatán, M. aurolineatus appears to be a common associate of echinoids dwelling within burrows. A small species of fish (max length 6 cm; Froese and Pauly, 2019), it easily fits within crevices bored by echinoids. Strasburg (1966) and Lieske and Myers (2002) reported that it is usually near echinoids, but no further details were provided. Juveniles and adults of G. filamentosus (max length 12 cm; Froese and Pauly, 2019) and juveniles of L. nuchipinnis (max length 23 cm; Froese and Pauly, 2019) may also dwell within echinoid burrows at Roatán, but both are much less common than M. aurolineatus.
All fishes lingered beside or under an echinoid's spines for extended periods. It is difficult to be certain whether the fishes associated directly with the echinoids for protection or simply sought shelter in the deepest recesses of crevices inhabited by echinoids and coincidentally appeared next to an echinoid. In either case they benefitted directly from the shelter provided by the burrowing activities of E. lucunter (McLean, 1967; Ogden, 1977; Hoskin and Reed, 1985; Asgaard and Bromley, 2008), and perhaps other species of echinoids which also burrow into rock (Bak, 1994).
The echinoid species differed greatly in their morphology (Hendler et al., 1995; Humann and DeLoach, 2002a). Diadema antillarum has the longest and thinnest spines, up to 200 mm long. Eucidaris tribuloides has thick and blunt spines of medium length, up to about 50 mm long. Echinometra lucunter and E. viridis have medium-length spines, up to about 30 mm long in the former species and 40 mm long in the latter. Tripneustes ventricosus and L. variegatus have short spines up to about 20 mm long. In our study, the more frequent association of fishes with the longest-spined species, D. antillarum, suggests that the fishes intentionally sought shelter beside echinoids to benefit from increased protection from predators. Hayes et al. (2016) found that decapod crustaceans also associated most frequently D. antillarum at Roatán.
Smaller fishes capable of retreating underneath or between the spines of echinoids are more likely to benefit from the protective spines of echinoids than larger fishes (Karplus, 2014). We never observed a fish with a total length > 10 cm associating with an echinoid and most were < 6 cm. Our observations of only juveniles of nine fish species associating with echinoids is consistent with several previous studies of fishes associating with echinoids in the Caribbean Sea. Only juveniles of two species of haemulin grunts, Haemulon plumierii and Haemulon flavolineatum, associated with D. antillarum at St. Croix (Helfman et al., 1983). Only juveniles of three fish species associated with D. antillarum at St. John (Townsend and Bologna, 2007): H. flavolineatum, the sciaenid drum Pareques acuminatus, and the tetraodontid puffer Canthigaster rostrata.
Of nine fish species associating with D. antillarum at Trindade Island of southeastern Brazil (Giglio et al., 2017), only juveniles of four fish species associated with the echinoid: the labrid wrasse Thalassoma noronhanum, the pomacentrid damselfish Chromis multilineata, the apogonid cardinalfish Apogon americanus, and the pomacentrid damselfish Stegastes fuscus. The adults of five fish species associating with D. antillarum at Trindade Island (Giglio et al., 2017) were all small: the labrisomid blenny Malacoctenus brunoi, the blenniid blenny Ophioblennius trinitatis, the gobiid goby Elacatinus pridisi, the cirrhitid hawkfish Amblycirrhitus pinos, and the gobiid goby Coryphopterus thrix.
Of the 11 fish species that we observed associating with echinoids, only M. aurolineatus had previously been reported associating with echinoids (Strasburg, 1966; Lieske and Myers, 2002). Although echinoids are preyed upon by at least 34 fish species in the Caribbean Sea and are the dominant food item for six species (Randall et al., 1964; Randall, 1967), small fishes pose no existential threat to echinoids when seeking shelter from potential predators among the sharp spines of echinoids (Karplus, 2014). However, some fishes associating with echinoids prey upon the tube feet and pedicillaria of their echinoid hosts (Pfaff, 1942; Briggs, 1955; Dix, 1969; Teytaud, 1971; Russell, 1983; Sakashita, 1992), which may also occur in the associations that we observed. None of the fish species in our study associated exclusively with echinoids or was specialized for associating with echinoids, indicating that the associations are facultative rather than obligatory.
ACKNOWLEDGMENTS
Our field work was funded by the Margaret Hughes Faculty Research Fund of Pacific Union College and by students taking the course Field Biology: Tropical Biology. We thank Dustin Baumbach, Stephen Dunbar, and Jimmy Miller for logistical assistance, and two anonymous reviewers for improving the manuscript.
REFERENCES
Asgaard, U. and R.G. Bromley. 2008. Echinometrid sea urchins, their trophic styles and corresponding bioerosion: 279-303. In: Wisshak, M. y L. Tapanila (Eds.). Current developments in bioerosion. Springer-Verlag, Berlin. 499 p. [ Links ]
Bak, R.P.M. 1994. Sea urchin bioerosion on coral reefs: place in the carbonate budget and relevant variables. Coral Reefs, 13: 99-103. [ Links ]
Birkeland, C. 1989. The influence of echinoderms on coral-reef communities: 1-79. In: Jangoux, M. y J.M. Lawrence (Eds.). Echinoderm studies 3. Balkema, Rotterdam, Netherlands. 90 p. [ Links ]
Briggs, J.C. 1955. A monograph of the clingfishes (order Xenopterygii). Stanford Ichthyol. Bull., 6: 1-216. [ Links ]
Bruce, A J. 1976. Shrimps and prawns of coral reefs, with special reference to commensalism: 37-94. In: Jones O. A. y R. Endean (Eds.). Biology and geology of coral reefs. Vol. 3. Biology 2. Academic Press, New York. 458 p. [ Links ]
Bruce, A.J. 1982. The shrimps associated with Indo-West Pacific echinoderms, with the description of a new species in the genus Periclimenes Costa, 1844 (Crustacea: Pontoniinae). Austral. Mus. Mem., 16: 191-216. [ Links ]
Carpenter, R.C. 1997. Invertebrate predators and grazers: 198-229. In: Birkeland, A. (Ed.). Life and death of coral reefs. Chapman and Hall, London. 536 p. [ Links ]
Castro, P. 1978. Settlement and habitat selection in the larvae of Echinoecuspentagonus (A. Milne Edwards), a brachyuran crab symbiotic with sea urchins. J. Exp. Mar. Biol. Ecol., 34: 259-270. [ Links ]
Chace, F.A. Jr. 1969. A new genus and five new species of shrimps (Decapoda, Palaemonidae, Pontoniinae) from the western Atlantic. Crustaceana, 16: 251-272. [ Links ]
Charteris, M. 2012. Caribbean reef life of the Bay Islands, Honduras. 2nd ed. Caribbean Reef Life, Roatan, Honduras. 347 p. [ Links ]
Clark, A.M. 1976. Echinoderms of coral reefs: 95-123. In: Jones, O. A. and R. Endean (Eds.). Biology and geology of coral reefs. Vol. 3. Biology 2. Academic Press, New York. 458 p. [ Links ]
Dix, T.G. 1969. Association between the echinoid Evechinus chloroticus (Val.) and the clingfish Dellichthys morelandi Briggs. Pac. Sci., 23: 332-336. [ Links ]
Eibl-Eibesfeldt, I. 1961. Eine Symbiose zwischen Fischen (Siphamia versicolor) und Seeigeln. Z. Tierpsychol., 18: 56-59. [ Links ]
Fricke, H.W. 1970. Ein mimetisches Kollektiv-Beobachtungen an Fischschwärmen, die Seeigel nachahmen. Mar. Biol., 5: 307-314. [ Links ]
Fricke, H.W. y M. Hentschel. 1971. Die Garnelen-Seeigel-Partnerschaft-eine Untersuchung der optischen Orientierung der Garnele. Z. Tierpsychol., 28: 453-462. [ Links ]
Froese, R. y D. Pauly (Eds.). 2019. FishBase. www.fishbase.org. 05/04/2019. [ Links ]
Giglio, V.J., M.L.F. Ternes, M.C. Barbosa, C.A.M.M. Cordeiro, S.R. Floeter and C.E.L. Ferreira. 2017. Reef fish associations with sea urchins in an Atlantic oceanic island. Mar. Biodiv., 18: 1833-1839. [ Links ]
Glynn, P.W. and I.C. Enochs. 2011. Invertebrates and their roles in coral reef ecosystems: 273-325. In: Dubinsky, Z. and N. Stambler (Eds.). Coral reefs: an ecosystem in transition. Springer, Berlin. 552 p. [ Links ]
Goldberg, W.M. 2013. The biology of reefs and reef organisms. University of Chicago Press, Chicago. 401 p. [ Links ]
Gould, A.L., S. Harii and PV. Dunlap. 2014. Host preference, site fidelity, and homing behavior of the symbiotically luminous cardinalfish, Siphamia ttubifer (Perciformes: Apogonidae). Mar. Biol ., 161: 2897-2907. [ Links ]
Hartney, K.B. and K.A. Grorud. 2002. The effect of sea urchins as biogenic structures on the local abundance of a temperate reef fish. Oecologia, 131: 506-513. [ Links ]
Hayes, F.E., M.C. Holthouse, D.G. Turner, D.S. Baumbach and S. Holloway. 2016. Decapod crustaceans associating with echinoids in Roatán, Honduras. Crust. Res., 45: 37-47. [ Links ]
Helfman, G.S., J.L. Meyer and W.N. McFarland. 1982. The ontogeny of twilight migration patterns in grunts (Pisces: Haemulidae). Anim. Behav., 30: 317-326. [ Links ]
Hendler, G., J.E. Miller, D.L. Pawson and PM. Kier. 1995. Sea stars, sea urchins, and allies: echinoderms of Florida and the Caribbean. Smithsonian Institution Press, Washington D.C. 390 p. [ Links ]
Hoskin, C.M. and J.K. Reed. 1985. Carbonate sediment production by the rock-boring urchin Echinometra lucunter and associated endolithic infauna at Black Rock, Little Bahama Bank. Symp. Ser. Underw. Res., 3: 151-161. [ Links ]
Humann, P. and N. DeLoach. 2002a. Reef creature identification: Florida, Caribbean, Bahamas. 2da ed. New World Publications, Jacksonville, FL. 447 p. [ Links ]
Humann, P . and N. DeLoach . 2002b. Reef fish identification: Florida, Caribbean, Bahamas. 3ra ed. New World Publications, Jacksonville, FL. 512 p. [ Links ]
Joseph, V.L., F.E. Hayes and N.A. Trimm, Jr. 1998. Interspecific selection of three potential urchin host species by the arrow crab Stenorhynchus seticornis (Crustacea, Decapoda, Brachyura). Carib. Mar. Stud., 6: 31-34. [ Links ]
Karplus, I. 2014. Symbiosis in fishes: the biology of interspecific partnerships. Wiley-Blackwell, West Sussex, UK. 449 p. [ Links ]
Keck, J. 2000. Instructor's guide: planning a field course. Roatán Institute for Marine Sciences, Roatán. 74 p. [ Links ]
Lachner, E.A. 1955. Inquilinism and a new record for Paramia bipunctata, a cardinal fish from the Red Sea. Copeia, 1955: 53-54. [ Links ]
Lallemant, H.G. and M. Gordon. 1999. Deformation history of Roatán Island: implications for the origin of the Tela Basin (Honduras): 197-218. En: Mann, P. (Ed.). Caribbean basins. Sedimentary basins of the world. Vol 4. Elsevier, Amsterdam, Netherlands. 696 p. [ Links ]
Lewis, J.B. 1956. The occurrence of the macruran Gnathophylloides minerii Schmitt on the spines of the edible sea-urchin Tripneustes esculentus Leske in Barbados. Bull. Mar. Sci., 6: 288-291. [ Links ]
Lieske, E. and R. Myers. 2002. Coral reef fishes: Caribbean, Indian Ocean and Pacific Ocean including the Red Sea. Rev. Ed. Princeton University Press, Princeton, NJ. 400 p. [ Links ]
Magnus, D.B.E. 1967. Ecological and ethological studies and experiments on the echinoderms of the Red Sea. Stud. Trop. Oceanogr., 5: 635-664. [ Links ]
McBirney, A. and M. Bass. 1967. Geology of Bay Islands, Gulf of Honduras: 229-243. In: McBirney, A. (Ed.). Tectonic relationships of northern Central America and the western Caribbean-the Bonacca Expedition. Am. As. Petr. Geol. Mem., 11.,355 p. [ Links ]
McLean, R.F. 1967. Erosion of burrows in beachrock by the tropical sea urchin, Echinometra lucunter. Can. J. Zool., 45: 586-588. [ Links ]
Monroy López, M. y O.D. Solano. 2006. Estado poblacional de Echinometra lucunter (Echinoida: Echinometridae) y su fauna acompañante en el litoral rocoso del Caribe colombiano. Rev. Biol. Trop., 53: 291-297. [ Links ]
Ogden, J.C. 1977. Carbonate-sediment production by parrot fish and sea urchins on Caribbean reefs. Amer. Assoc. Petrol. Geol. Stud. Geol., 4: 281-288. [ Links ]
Patton, W.K., R.J. Patton and A. Barnes. 1985. On the biology of Gnathophylloides mineri, a shrimp inhabiting the sea urchin Tripneustes ventricosus. J. Crust. Biol., 5: 616-626. [ Links ]
Pfaff, J.R. 1942. On a new genus and species of the family Gobiesocidae from the Indian Ocean, with observations on sexual dimorphism in the Gobiesocidae, and on the connection of certain gobiesocids with echinoids. Vidensk. Medd. Dansk Naturh. Foren., 105: 413-422. [ Links ]
Randall, J.E. 1967. Food habits of reef fishes of the West Indies. Stud. Trop. Oceanogr ., 5: 665-847. [ Links ]
Randall, J.E., R.E. Schroeder and W.A. Starck, II. 1964. Notes on the biology of the echinoid Diadema antillarum. Carib. J. Sci., 4: 421-433. [ Links ]
Robertson, D.R. and J. Van Tassell. 2015. Shorefishes of the greater Caribbean: online information system. Version 1.0, Smithsonian Tropical Research Institute, Balboa, Panamá. biogeodb.stri.si.edu/caribbean/en/pages . 05/04/2019. [ Links ]
Russell, B.C. 1983. The food and feeding habits of rocky reef fish of north eastern New Zealand. New Zeal. J. Mar. Fresh. Res., 17: 121-145. [ Links ]
Sakashita, H. 1992. Sexual dimorphism and food habits of the clingfish, Diademichthys lineatus, and its dependence on host sea urchin. Environ. Biol. Fishes, 34: 95-101. [ Links ]
Schoppe, S. 1991. Echinometra lucunter (Linnaeus) (Echinoidea, Echinometridae) als Wirt einer komplexen Lebensgemeinschaft im Karibischen Meer. Helgol. Meeresunters., 45: 373-379. [ Links ]
Schoppe, S. and B. Werding. 1996. The boreholes of the sea urchin genus Echinometra (Echinodermata: Echinoidea: Echinometridae) as a microhabitat in tropical South America. Mar. Ecol., 17: 181-186. [ Links ]
Steneck, R.S. 2013. Sea urchins as drivers of shallow benthic marine community structure. Develop. Aquacult. Fish. Sci. 38: 195-212. [ Links ]
Strasburg, D.W. 1966. Observations on the ecology of four apogonid fishes. Pac. Sci ., 20: 338-341. [ Links ]
Tamura, R. 1982. Experimental observations on the association between the cardinalfish (Siphamia versicolor) and the sea urchin (Diadema setosum). Galaxea, 1: 1-10. [ Links ]
Teytaud, A.R. 1971. Food habits of the goby, Ginsburgellus novemlineatus, and the clingfish, Arcos rubiginosus, associated with echinoids in the Virgin Islands. Carib. J. Sci ., 11: 41-45. [ Links ]
Townsend, T. and P.A.X. Bologna. 2007. Use of Diadema antillarum spines by juvenile fish and mysid shrimp. Gulf Carib. Res., 19: 55-58. [ Links ]
Wells, S.M. (Ed). 1988. Coral reefs of the world. Vol. 1. Atlantic and Eastern Pacific. United Nations Environment Programme and International Union for Conservation of Nature and Natural Resources, Cambridge, UK. 373 p. [ Links ]
Zar, J.H. 2010. Biostatistical analysis. 5th ed. Prentice Hall, Upper Saddle River, NJ. 944 p. [ Links ]
Received: December 27, 2018; Accepted: April 09, 2019











 texto en
texto en