Introduction
Eosinophilic esophagitis is a chronic inflammatory condition immunologically mediated, distinguished by esophageal dysfunction symptoms, local endoscopic inflammatory alterations, and an eosinophilic infiltrate confined to the esophagus. Its diagnosis encompasses clinical, endoscopic, and histological findings. Symptoms typically emerge during school age but can manifest at any age1,2. Early diagnosis and prompt treatment are critical for improving the prognosis by halting disease progression and preventing secondary complications arising from esophageal dysfunction and tissue remodeling. Recent years have seen various pediatric management strategies published, notably including high-dose proton pump inhibitors, topical corticosteroids, and exclusion diets. Despite these approaches, a significant fraction of patients, at least one-third, may not achieve remission with initial treatment, presenting a therapeutic challenge to gastroenterologists and pediatric allergists3,4.
The disruption of the esophageal barrier plays a pivotal role in eosinophilic esophagitis pathophysiology, facilitating the penetration of food allergens. This initiates an immune response that is not mediated by immunoglobulin E (IgE), further amplified by the production of thymic stromal lymphopoietin (TSLP), which activates lymphocytes to produce a type 2 inflammatory response (Th2). As a result, pro-inflammatory cytokines are generated, promoting eosinophil recruitment to the esophageal epithelium and their degranulation, leading to inflammation and fibrosis2,5.
Diagnosing eosinophilic esophagitis necessitates clinical, endoscopic, and histological criteria. The onset of symptoms typically occurs in school-aged children, with incidence peaking during adolescence and early adulthood. Clinical manifestations vary based on the age of onset and may include dysphagia, upper abdominal pain, and food impaction3,6. Upper gastrointestinal endoscopy (UGIE) allows for the evaluation of the esophageal appearance and biopsy collection to confirm eosinophilic infiltration of the mucosa. Histological examination commonly reveals acanthosis, basal cell hyperplasia, and eosinophilic inflammatory infiltrate, with a requirement of more than 15 eosinophils per high power field (HPF), thus recommending at least six biopsies across the esophagus’s thirds. Chronic inflammation can lead to fibrostenosis, characterized by concentric ring lesions and esophageal stenosis7.
The type 2 inflammatory response involves the secretion of interleukins (IL) 4, IL-13, and IL-5, leading to an increased eosinophilic infiltrate in the tissue, which contributes to tissue remodeling. This inflammatory pattern is common to atopic dermatitis, asthma, allergic rhinitis, and eosinophilic esophagitis. IL-4 encourages a shift towards Th2 lineage and prompts B lymphocytes to produce IgE. Both IL-4 and IL-13 enhance cellular migration to inflammation sites, whereas IL-13 also increases mucus production and smooth muscle contractility, thereby facilitating mast cell proliferation, antigen recognition, degranulation, and enhancing the inflammatory response5,8.
Dupilumab is a humanized monoclonal antibody that serves as a dual inhibitor of the alpha chain of the IL-4 and IL-13 receptors, having a significant impact on the Th2 inflammatory cascade9,10. A clinical trial conducted on adolescents and adults with eosinophilic esophagitis has shown the efficacy and safety of dupilumab. The trial involved administering a loading dose of 600 mg, followed by 300 mg administered subcutaneously (SC) weekly, which resulted in improvements in clinical, endoscopic, and histological parameters over 12 weeks of treatment compared to a placebo11. For pediatric patients younger than 12 years, substantial studies on the safety and efficacy of dupilumab in treating eosinophilic esophagitis are yet to be conducted, but it is viewed as a promising therapy based on its pathophysiological rationale8,12.
Clinical case 1
We present the case of a 9-year-old female with a history of allergic rhinitis, asthma, atopic dermatitis, and IgE-mediated food allergy. At the age of 6, due to a failure to thrive, a UGIE was performed, revealing a normal-looking esophagus (EREFS 0), with esophageal biopsies confirming the diagnosis of eosinophilic esophagitis. This led to the initiation of a dietary restriction eliminating milk; however, the patient lost follow-up due to the SARS-CoV-2 pandemic. At 8 years old, she presented again with symptoms of impaction and chest pain. A repeat UGIE showed EREFS 5, characterized by exudates, edema, furrows, and a crepe-paper appearance of the mucosa (Table 1). The esophageal biopsies indicated 30 eosinophils per HPF, acanthosis, and basal hyperplasia. Consequently, treatment was augmented with proton pump inhibitors (PPIs) at a dosage of 2 mg/kg/day and swallowed fluticasone at 250 μg every 12 hours, yet no improvement was observed. Due to the clinical severity and lack of response, specific IgE testing for food allergens was conducted, revealing sensitization to multiple allergens: casein, lactoalbumin, lactoglobulin, wheat, soy, pineapple, peanuts, and oats. A dietary restriction for foods with positive IgE was initiated, but the response was irregular, and the patient and her mother faced challenges in adhering to the dietary exclusion, particularly during school hours.
Table 1 Case 1: Evolution of EREFS Scores and Histopathological Findings
| Age | 6 Years | 8 Years | 10 Years | |
|---|---|---|---|---|
| UGIE | EREFS | 0 | 5 | 1 |
| Histology | Acanthosis | Not reported | Present | Present |
| Basal Hyperplasia | Not reported | Present | Present | |
| No. of Eosinophils x HPF | 18 | 30 | 13 | |
| Dupilumab therapy |
HPF: High Power Field. Author’s own research.
Despite the patient’s nutritional supplementation with an elemental formula (Neocate®), she was found to have low weight, short stature, and bone densitometry indicative of osteopenia (T-score -2 SD). Managing her asthma, rhinitis, and dermatitis proved challenging, with clear evidence of dust mite sensitization. Given these difficulties, treatment was initiated with dupilumab 300 mg subcutaneously (SC) every four weeks. Remarkably, after two months of therapy, the patient experienced complete resolution of her gastrointestinal symptoms.
Six months into the biological therapy regimen, a follow-up UGIE revealed only mild furrowing (EREFS 1). Histological analysis of the proximal, middle, and distal thirds of the esophagus showed fewer than 15 eosinophils per HPF, (Table 1). Furthermore, after six months on dupilumab, reevaluation of specific IgE levels to foods demonstrated a reduction of over 50% for casein, peanuts, and wheat. This positive outcome prompted the cautious reintroduction of these foods into the patient’s diet (Table 2). The patient currently reports complete gastrointestinal symptom resolution and has achieved excellent control over her allergic comorbidities, leading to a phased reintroduction of previously restricted foods.
Table 2 Specific IgE Levels to Foods Before and Six Months After Starting Dupilumab
| Allergen (IgE kUI/L) | Patient 1 (Beginning of Treatment) | Patient 1 (6 Months into Treatment) | Patient 2 (Beginning of Treatment) | Patient 2 (6 Months into Treatment) |
|---|---|---|---|---|
| Casein | 7.82 | 1.6 | 8.92 | 10.4 |
| Alpha-lactalbumin | 2.55 | 1.75 | 33.3 | 17.4 |
| Beta-lactoglobulin | 0.78 | 0.54 | 34.8 | 20.9 |
| Egg yolk | - | - | 40.7 | 24.3 |
| Egg white | - | - | 60.3 | 34.9 |
| Peanut | 4.72 | 0.2 | 4.06 | 2.25 |
| Wheat | 3.6 | 0.1 | 1.52 | 0.8 |
| Avocado | - | - | 2.17 | 0.74 |
| Orange | - | - | 5.17 | 2.41 |
| Soy | - | - | 1.87 | 0.86 |
IgE: Immunoglobulin E. Author’s own research.
Clinical case 2
This case involves an 8-year-old male patient with a background of asthma, allergic rhinitis, and IgE-mediated food allergy. At 8 years, due to failure to thrive and extensive dietary restrictions, a UGIE and biopsy identified more than 20 eosinophils per HPF, alongside exudates, rings, and furrows (EREFS 4) (Figure 1A). Elevated specific IgE levels were documented for casein, serum albumins, egg (both yolk and white), fish, sesame, avocado, orange, strawberry, soy, and wheat. Subsequently, a diet restricting milk, egg, and fish was initiated, along with swallowed fluticasone at a dosage of 250 μg every 12 hours and PPI at a dose of 2 mg/kg/day, albeit without clinical improvement. Subsequent UGIE revealed an eosinophil count exceeding 50 per field.
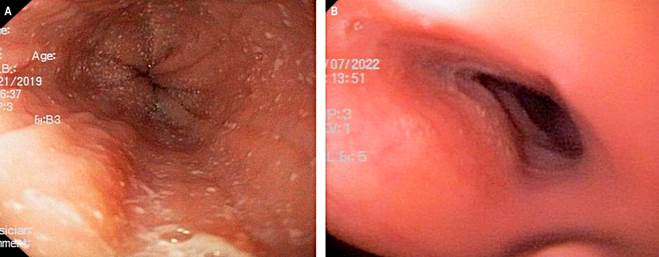
Figure 1 Case 2. A. Esophagogastroduodenoscopy showing an EREFS 4 esophagus, marked by the presence of exudates, rings, and furrows. B. Esophagogastroduodenoscopy showing an EREFS 0 esophagus, marked by the absence of exudates, rings, or furrows. Source: Author’s File.
The patient exhibited clinical symptoms indicative of a mixed food allergy, manifesting as hives, vomiting, and diarrhea following accidental poisoning, alongside persistent abdominal pain and food impaction. Consequently, the parents opted for homeschooling, avoided dining in restaurants, and eschewed attendance at social gatherings, leading to isolation and depressive symptoms in the patient. Given the ongoing nature of these symptoms, coupled with malnutrition and stunted growth, treatment was commenced with dupilumab 300 mg administered subcutaneously every four weeks. Remarkably, a full resolution of symptoms was observed within two months of initiating treatment. A follow-up UGIE conducted six months after the start of dupilumab therapy revealed an EREFS score of 0 (Figure 1B), with biopsy results indicating a maximum eosinophil count of 15 per HPF throughout the esophagus (Table 3).
Table 3 Case 2: Evolution of EREFS Scores and Histopathological Findings
| Age | 6 Years + 9 Months | 7 Years | 8 Years | 8 Years + 9 Months | |
|---|---|---|---|---|---|
| UGIE | EREFS | 4 | 5 | 4 | 0 |
| Histology | Acanthosis | Present | Present | Present | Absent |
| Basal Hyperplasia | Present | Present | Present | Absent | |
| No. of Eosinophils x HPF | > 20 | 100 | 50 | < 15 | |
| Dupilumab therapy |
HPF: High Power Field. Author’s own research.
Following six months of dupilumab treatment, reassessment of specific IgE levels to various foods indicated a reduction of over 50% in most foods. Notably, however, IgE levels for casein exhibited an increase, necessitating the continued dietary restriction against milk (Table 2). Encouragingly, the patient successfully reintroduced avocado, orange, peanut, and soy into his diet and was able to resume in-person education, with a concurrent resolution of depressive symptoms.
Discussion
In this report, we detail the experiences of two patients with a confirmed atopic march, suffering from eosinophilic esophagitis that was unresponsive to dietary interventions, swallowed steroids, and PPIs. These patients exhibited significant clinical improvement following the administration of dupilumab 300 mg subcutaneously (SC) every four weeks, a dosage guideline adapted from treatments for asthma and atopic dermatitis. It is noted that around 60% of individuals with eosinophilic esophagitis find relief through conventional treatments including PPIs, swallowed corticosteroids, dietary restrictions, and esophageal dilations. Yet, there exists a subset of patients who continue to experience eosinophilic inflammation of the esophagus despite comprehensive management efforts3,7.
The advancing comprehension of immunological pathways implicated in allergic diseases has facilitated the emergence of monoclonal medications targeted at the Th2 inflammatory pathway. Dupilumab has been approved for use in severe atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps12. In a significant development, the Food and Drug Administration (FDA) in May 2022 approved dupilumab for individuals aged 12 years and older with eosinophilic esophagitis. Its application has been associated with improvements in dysphagia, reductions in eosinophil counts, enhancements in the EREFS endoscopic scale, and improved esophageal distensibility in comparison to placebo. Although the recommended dosage for adolescents and adults is established as a 600 mg SC loading dose followed by 300 mg SC weekly13, the optimal dose for children under 12 remains undetermined. Presently, British consensus guidelines in gastroenterology, hepatology, and pediatric nutrition advocate for the consideration of dupilumab in treating eosinophilic esophagitis, especially in cases where initial treatments fail. Positive effects of dupilumab have also been documented in other eosinophilic gastrointestinal disorders6,8,12.
Our cases received doses recommended for their weight and age, aligned with guidelines for asthma and atopic dermatitis, due to the absence of studies specifically addressing eosinophilic esophagitis in children under 12 years at the time treatment commenced. Remarkably, clinical remission was observed prior to the administration of the third dupilumab dose, with subsequent histological remission confirmed via control endoscopy after six months of therapy. The patients also showed improved management of their asthma, rhinitis, and dermatitis and were able to commence immunotherapy for aeroallergen sensitivities as part of their comprehensive allergy treatment plan. Pain at the injection site was the sole adverse effect reported.
Spekhorst and colleagues (2022) described a cohort of 125 individuals with atopic dermatitis and food allergies who exhibited decreased specific IgE levels to foods after receiving dupilumab for atopic dermatitis14. Similarly, Rial and colleagues observed that patients with atopic dermatitis and IgE-mediated allergies to corn and nuts did not report symptoms following accidental ingestion after being treated with dupilumab15. In our patients, a decrease of over 50% in specific IgE levels to a majority of the restricted foods was recorded, enabling the cautious reintroduction of these foods with suitable clinical tolerance. These observations suggest that dupilumab may facilitate tolerance induction and mitigate the severity of allergic reactions to food by persistently inhibiting Th2 activity. Notably, in patient 2, an increase in casein levels was observed, hinting at the potential of dupilumab to uncover true food allergies.
Determining the optimal dupilumab management strategy for eosinophilic esophagitis in pediatric patients remains an area of active investigation. This includes clarifying whether induction doses are necessary, identifying the appropriate therapeutic dose, establishing treatment duration, and assessing the drug’s long-term efficacy in maintaining disease remission.
Conclusions
Eosinophilic esophagitis has been identified as a recently characterized condition for which there is not yet a universally effective treatment. Various therapeutic approaches exist, each yielding varying degrees of clinical, endoscopic, and histological success.
It is crucial to educate the medical team on the importance of timely diagnosis and intervention to prevent disease progression and avert complications, thereby ensuring an improved quality of life for affected patients.
Treating these patients, who often present with multiple comorbidities rooted in similar pathophysiological mechanisms, necessitates a coordinated approach within specialized centers experienced in managing such complex cases with multidisciplinary teams. Dupilumab stands out as a safe and efficacious therapeutic option that merits consideration for patients with severe or extensive Th2 inflammation and those unresponsive to traditional clinical management strategies for eosinophilic esophagitis.











 texto em
texto em 



