Introduction
Patent foramen ovale (PFO) is a persistent fetal connection between the right and left atria caused by incomplete closure of the atrial septum. When the pressure in the right atrium surpasses that of the left atrium (for example, with physical exertion or the Valsalva maneuver), blood will flow from right to left. Autopsies showed that the foramen ovale remains dynamically permeable in approximately one fourth of the general population 1. Therefore, PFO is the most common congenital heart abnormality. Of all the septal defects, PFO causes 95% of shunts in adults 2,3. For most people, a PFO will be asymptomatic their whole lives. However, ever since it was first described by Cohnheim et al. in 1877, there has been growing recognition of PFO as a potential mediator of systemic emboli. Today, percutaneous closure of septal defects has spread widely; given its relative ease of implantation, it is practiced widely and, in some centers, has replaced the surgical approach 4.
Various publications have shown a significant association between PFO and cryptogenic stroke, and, while controversy persists in some scenarios, there are data favoring percutaneous closure of PFOs 5. For many years we lacked quality evidence to make clinical value judgements in the face of reasonable uncertainty regarding PFO closure, and although we currently have encouraging data in favor of percutaneous PFO closure, there are some articles in which no net clinical benefit is obtained 6.
In our country, despite the growing accessibility of percutaneous septal defect closure, there are few descriptions of the clinical outcomes, complications and beneficiaries of this intervention, which necessitates an understanding of aspects related to the specific characteristics of our patients. The objective of this paper is to study the immediate and long-term follow up outcomes of a consecutive series of patients with PFOs closed percutaneously at a tertiary referral center.
Materials and methods
An observational, descriptive study was carried out in a retrospective cohort of patients with PFOs who consecutively underwent percutaneous closure at a tertiary reference center from January 1, 2016, to September 1, 2021, due to a cryptogenic cerebrovascular accident (CVA). Cryptogenic CVA was defined as a transient or sustained cerebrovascular accident with an unknown or uncertain etiology based on the clinical data and diagnostic test results (including at least an electrocardiogram, 24-hour Holter test, neck vessel Doppler and echocardiography), and which, in addition, did not meet the criteria for a lacunar CVA (small-diameter lesions, no greater than 1 mm, caused by occlusion in the territory of the penetrating arteries of the brain). The inclusion criteria were patients over the age of 18 who had undergone percutaneous PFO closure during the study period.
Patients with incomplete medical charts and patients who could not be contacted for the necessary interview to identify the outcomes of interest, were excluded. A convenience sample was taken from the noted time period to analyze the total number of procedures performed. A chart review was done to identify the general interest variables like age, weight, height, and body mass index. Past medical history was also reviewed, as well as the presence of smoking, obesity, hypertension, diabetes mellitus or hypercholesterolemia, and additional risk factors like alcohol and the use of oral contraceptives. The number of traditional cardiovascular risk factors was determined, along with a history of migraines as well as baseline echocardiographic characteristics like atrial septal aneurysms, baseline bubble passing, its relationship with the Valsalva maneuver, and the presence or absence of significant bubble passing. Finally, variables related to the intervention like the closure device used and the size of the atrial septal defect.
The variables were collected in a predesigned Microsoft Excel® database and double checked to minimize data entry errors. The data recorded in the follow up medical chart were used for the follow-up clinical outcomes, along with a telephone call to inquire about the patient>s vital status 30 days and six months after device implantation. The clinical characteristics were analyzed individually, and complex anatomy was evaluated, defined for this article>s purposes as atrial septal aneurysm, a greater than 10 mm length of the defect or significant baseline bubble passage. The RoPE score was reviewed (a scale which has helped meet the challenge of identifying which cryptogenic CVAs may be attributed to an FOP), and the risk was stratified as low probability (less than seven points) or high probability (equal to or greater than seven) 7.
Immediate (<72 hour) outcomes related to the procedure were evaluated, such as cardiac tamponade, atrial fibrillation, allergic reactions to the contrast medium, intra-chamber thrombi, pulmonary emboli, deep vein thromboses, hematomas, puncture site infections, fistulas, vascular aneurysms or failed closure. Early and late clinical outcomes like the onset of atrial fibrillation, recurrent CVAs, neurological death or death from other causes were also evaluated. For this article, we defined early complications as the clinical outcomes presenting within less than six months, and late complications as those occurring after this time.
Serious adverse events (SAEs) were defined as those leading to death, requiring hospitalization or prolonging hospitalization, those that were potentially fatal, and those resulting in persistent or significant disability/invalidity or in a congenital anomaly/defect (death, major bleeding, atrial fibrillation, atrial flutter, device dislodgement, device thrombosis, aortic dissection, pulmonary embolism, cardiac perforation, hematoma at the puncture site, aneurysm at the puncture site, residual shunt, infective endocarditis, the need for a new intervention, deep vein thrombosis or pulmonary embolism). Inpatient and follow-up mortality were also evaluated using the medical chart or a telephone call. The two primary safety evaluation criteria were all-cause 30-day mortality and the rate of disabling CVA at 30 days and six months. Other variables included important adverse events, cardiac events, the need for cardiac surgery and hemorrhages. The secondary efficacy evaluation criteria were the rate of success and the complications. The study was approved by the Ethics Committee and Research Committee. All procedures were performed according to institutional guidelines, and the informed consent requirement did not apply due to the study>s retrospective design.
The clinical and demographic characteristics obtained from the medical chart were described. Qualitative variables are presented as percentages and quantitative variables as mean and standard deviation. Quantitative variables were evaluated with the Shapiro-Wilk test to determine if they were normally distributed. A Kaplan-Meyer analysis was done to determine the survival values of the patient cohort analyzed. The safety outcomes of patients undergoing implantation were analyzed, and the efficacy outcomes were analyzed in patients with successful implants. All statistical analyses were done using SAS version 9.2 software.
Results
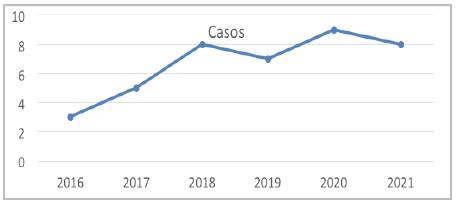
From January 1, 2016, to September 1, 2021, 40 percutaneous PFO closures were performed. This article includes all the patients who underwent the procedure, with the Amplatzer® device used in all cases (Figure 1). A mean follow-up of 2.3 years was obtained (minimum of 47 days, maximum of 5.4 years). Clinical follow up was achieved for all patients. The mean age was 43 ± 13.6 years, 7% were over 60, 72.5% were women, 25% were hypertensive, 20% were diabetic, and 10% of the patients had a history of migraines (Table 1). For 75% of the patients, the indication for closure was a CVA at the index event, for 10% it was a history of CVAs, and for 12.5% it was due to characteristics of the defect. The mean RoPE score was six points and 50% had a score greater than or equal to seven. Thirty-two percent of the cases had complex anatomy.
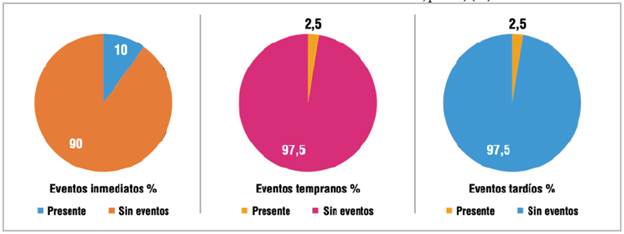
Of all the cases performed, three (7.5%) had serious adverse events (one immediate, one early and one late), and four had immediate complications (10%) (three failed closures and one arteriovenous vascular access aneurysm), which accounted for all the immediate events. The failed closures were due to discrepancies between the pre-procedure echocardiogram and visualization of the defect during the procedure.
On follow up, one case (2.5%) had an early event (atrial fibrillation) and 2.5% had a late CVA recurrence (Figure 2). There were no neurological deaths during follow up. The most commonly used antiplatelet therapy at discharge was the combination of acetylsalicylic acid and clopidogrel in 80% of cases, and there was 100% survival throughout follow up.
Discussion
Patent foramen ovale has a high prevalence in the general population. Autopsies in the United States have shown an incidence of 20-26%, and it can be found in up to 50% of patients over the age of 55 with cryptogenic CVAs 5,6,8-11.
September 14, 2017, marks a significant milestone in the treatment of patients with PFO. The New England Journal of Medicine (NEJM) published three articles simultaneously which sought to change the trend toward percutaneous closure in adults under 60, showing a decreased rate of CVA recurrence in those assigned to PFO closure combined with antiplatelet therapy versus those assigned to antiplatelet therapy alone. The number needed to treat (NNT) to prevent a cerebrovascular accident in five years was 42 patients in the RESPECT trial and 20 in the CLOSE trial, and to prevent one event in the two-year follow up was 28 patients in the REDUCE trial 12-14. The presence of a PFO has been accepted as a possible cause of embolic CVAs, especially in cases where it is associated with an atrial septal aneurysm (ASA) 15. In our population, 32% of the patients had complex anatomy and 7% had ASA, which is greater than the 1% reported in autopsy series and the 2.2% in a population-based transesophageal echocardiogram study 16,17. In 12.5% of the cases, the motivation for closure was the defect's characteristics. In this regard, Guillaume et al. evaluated the respective influence of the size of the PFO and the state of the ASA on cryptogenic CVA recurrence.
The authors grouped the data of individual patients from two prospective observational studies and the medical treatment arms of two randomized trials, in which the size of the shunt and ASA status were evaluated through independent readings of echocardiography images.
Out of 898 patients in this cohort, 19.8% had an ASA with a large PFO, 7.9% had an ASA with a small PFO, and 44.2% had a large PFO without an ASA. They used a model which considers age, hypertension, antithrombotic treatment and PFO anatomy; an ASA was independently associated with recurrent CVA (adjusted risk index: 3.27; 95% confidence interval [CI]: 1.82-5.86; p <0.0001), while a large PFO was not (average adjusted risk ratio between the studies: 1.43; 95% CI: 0.50-4.03; p=0.50) 18.
Table 1 Patient characteristics.
| General data | Frequency | Percentage (%) |
|---|---|---|
| Age range | ||
| 18-25 | 4 | 10 |
| 26-35 | 6 | 15 |
| 36-45 | 11 | 27 |
| 46-55 | 9 | 22 |
| 55-65 | 7 | 17 |
| >65 | 3 | 7 |
| Sex | ||
| Male | 11 | 27.5 |
| Female | 29 | 72.5 |
| History | ||
| HTN | 10 | 25 |
| DM | 8 | 20 |
| Smoking | 3 | 7,5 |
| Dyslipidemia | 2 | 5 |
| Obesity | 2 | 5 |
| Migraine with aura | 2 | 5 |
| More than two cardiovascular risk factors | 8 | 20 |
| Indication for PFO closure | ||
| Prior CVA | 4 | 10 |
| Index CVA at the time of closure | 30 | 75 |
| Transient ischemic attack | 1 | 2.5 |
| Defect characteristics | 5 | 12.5% |
| Procedure guidance | ||
| Transesophageal Echocardiogram | 39 | 97.5 |
| Fluoroscopy | 40 | 100 |
| Device used | ||
| Amplatzer® | 40 | 100 |
This highlights the importance of the PFO characteristics when determining closure, since despite the high prevalence described, paradoxical embolism is rare and is generally assumed more than can be proved 19. In an observational study of 139 patients with major pulmonary embolism, Konstantinides et al. showed that patients with PFO were more likely to die (44 versus 13%, p=0.02) and have a CVA (13 versus 2%, p=0.02) or peripheral embolism (15 versus 0%, p=0.01), with the presence of an emergent PFO being an independent predictor of mortality 20.
Table 2 Serious adverse events compared by clinical trial.
| Our experience (40 cases) (%) | RESPECT trial intervention group (499) (%) | CLOSE trial intervention group (238) (%) | GORE REDUCE intervention group (441) (%) | |
|---|---|---|---|---|
| Total serious adverse events | 7.5 | 4.2 | 35 | 23 |
| Atrial fibrillation/ flutter | 2.5 | 3 | 4.6 | 6.6 |
| Recurrent CVA | 2.5 | 2 | 0 | 4.7 |
In Europe, the annual risk attributed to paradoxical emboli has been estimated as 28 for every 100,000 people with PFO per year 21. Regarding the main outcomes, we reported 7.5% serious adverse events, which is similar to the RESPECT data 12 which reported 4.2% SAEs and is significantly less than the CLOSE investigators' 35% 14 and the 23% for GORE REDUCE 13 in the intervention groups. For immediate events, we reported 7.5% failed closures and one vascular complication (2.5%).
We reported a single case of early complications (2.5%), mainly due to the onset of atrial fibrillation within the first six months. In the large clinical trials, the rate of atrial fibrillation was greater in the PFO closure group than in the antiplatelet group; 6.6% in REDUCE, 3% in RESPECT, 4.6% in CLOSE, and 2.9% in the PC trial 10,12-14, and only two cases in DEFENSE-PFO 22. In our cohort's follow up, recurrent CVA occurred in one case (2.5%) as a late event (>6 months). In the RESPECT trial, recurrence of indeterminate cause occurred in 10 patients in the PFO closure group and 23 patients in the medical treatment group (risk ratio, 0.38; 95% CI, 0.18-0.79; p=0.007) 12.
In the CLOSE research group, there were no CVAs among the 238 patients in the PFO closure group, while CVAs occurred in 14 of the 235 patients in the antiplatelet-alone group (hazard ratio, 0.03; 95% CI, 0 to 0.26; p<0.001) 14. In GORE REDUCE, the incidence of new brain infarcts was 18 patients (4.7%) in the intervention group versus 19 patients (10.7%) in the medical management group (relative risk, 0.44; 95% CI, 0.24 - 0.81; p=0.02) 13 (Table 2).
The main limitations of our study include its retrospective nature, the use of a non-randomized sample, a small sample size, and the lack of a control group. We used the echocardiogram reports in the medical charts to categorize the patients, without reevaluating the images.
The echocardiographic finding definitions were not predetermined. For example, the definitions of interatrial septal aneurysm vary widely in the literature (10 to 15 mm total curvature, 10 to 15 mm in one direction or the other, etc.). The evaluation of early and late complications was done by telephone in most cases. More studies are needed to determine long-term outcomes in a larger patient population.
Conclusions
The latest randomized clinical trials have shown the benefit of percutaneous PFO closure in patients with cryptogenic cerebrovascular events. In our experience, we have found a low percentage of immediate complications; those described were mainly related to vascular access. There was a low rate of early and late events on follow up, and we reported 100% survival during follow up.











 texto em
texto em