Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Colombian Journal of Anestesiology
versão impressa ISSN 0120-3347
Rev. colomb. anestesiol. v.38 n.1 Bogotá jan./mar. 2010
Phentanyl PK/PD, a valid drug
Luis Alberto Tafur*, Ana Milena Serna**, Eduardo Lema***
* Médico anestesiólogo, Universidad del Valle, Hospital Universitario del Valle; Clínica Visual y Auditiva, Instituto para Niños Ciegos y Sordos del Valle del Cauca, Cali, Colombia
** Médica anestesióloga, Universidad del Valle, Hospital Universitario del Valle, Cali, Colombia
*** Médico anestesiólogo, Universidad del Valle, Hospital Universitario del Valle; Clínica Visual y Auditiva, Instituto para Niños Ciegos y Sordos del Valle del Cauca; docente, Departamento de Anestesiología, Universidad del Valle, Cali, Colombia
Recibido: noviembre 8 de 2009. Enviado para modificaciones: diciembre 1 de 2009. Aceptado: febrero 16 de 2010.
ABSTRACT
With the advent of the new opioids in the anesthetic drug market, we may think that we have molecules available to replace the legendary fentanyl. However, studying its pharmacodynamic and pharmacokinetic properties, fentanyl can still be appropriately used to take advantage of its excellent analgesia and safety, comparable to that of other opioids when administered in accordance with pharmacokinetic models.
We must not forget however that several drugs frequently used during anesthesia (midazolam, dexametasone, prednisolone, ketamin, etc.), may impact the metabolism of fentanyl on account of the cytochrome P450 3A4 enzyme.
The knowledge and judicious application of pharmacokinetic models serve to estimate plasma concentrations in order to ensure the best analgesic conditions associated to synergies with the frequent use of hypnotics such as propofol and desfluorane. fentanyl is best used for specific indications such as extended surgical procedures (over 120 minutes) or procedures that stimulate the patient´s pain perception.
Key words: fentanyl, pharmacokinetics, pharmacology, nomograms, intravenous anesthesia (Source: MeSH, NLM)
INTRODUCTION
Opium was the name given by Hippocrates around 400 B.C. and it means juice. Opioids are a group of drugs - whether derived from opium or not - that have a selective affinity for opioid receptors, with a morphine-like activity. The term opiate is used to refer to any opium-derived substance, regardless of its morphine-like activity (1). Anesthesia has evolved hand-in-hand with the development of new drugs with exceptional properties. However, the judicious knowledge of the pharmacokinetics and the pharmacodynamics of their predecessor allow us to continue using them safely and efficiently.
OBJETIVE
To review the pharmacokinetic and pharmacodynamic properties of fentanyl in order to provide the reader with the tools to select fentanyl as a valid option.
A PubMed search of all the articles on the pharmacokinetics and pharmacodynamics of opioids was completed, and the relevant ones were included in the review.
Opioids can be divided into three groups: the first are the natural opium alkaloids derived from phenanthrene (morphine and codeine) and the byproducts of Benzylisoquinoline (papaverine and thebaine).
The second group is the semi-synthetic opioids including those derived from morphine (oximorphone and hydromorphone), the thebaine byproducts (bupremorphine and hydromorphone) and codeine byproducts (tramadol).
The third group includes the synthetic opioids such as morphinanes (levorphanol, nalbuphine, naloxone and naltrexone), the Phenylethylamines (methadone, propoxifen) and the phenylpiperidines (meperidine, fentanyl, sufentanyl, alfentanyl and remifentanyl).
The potency of opioids follows the acronym "MEMOALFERESU" in descending orden; the least potent is meperidine and the most potent are morphine, alfentanyl, fentanyl, remifentanyl and sufentanyl. In average, fentanyl is 100 times more potent than morphine and remifentanyl is four times more potent than potent than fentanyl (2)
8 % of fentanyl is eliminated unchanged and 6% is excreted in the urine while 2 % is excreted in the feces. Over 80 % of the fentanyl in the body is metabolized by the cytochrome P450 3A4 present in the liver and in the gut. 99% of the fentanyl metabolites belong to norfentanyl (inactive metabolite) of which 76% is excreted in the urine and 8 % in the feces (2,3,4).
When fentanyl is administered, we must keep in mind the drugs that inhibit cytochrome P450 3A4, since this would result in a lower production of norfentanyl and greater fentanyl availability that changes completely the pharmacokinetics and the pharmacodynamics of the drug (5).
Labroo et al. (6) showed that midazolam reduced the production of norfentanyl by nearly 95 %, both in the liver and in the intestinal lumen. Other drugs affecting this enzyme and that we manage on a daily basis are: dexametasone, prednisolone, ketamine, antidepressants and alfentanyl, inter alia.
With the generalized use of fentanyl in the 80s, cases of respiratory depression in the post-anesthesia care units began to be reported. One likely explanation was fentanyl´s re-absorption by the intestinal lumen after being excreted in the stomach (7). The studies endorsing this hypothesis were done in animals but there are no reports in the literature documenting elevated fentanyl concentrations in the intestinal lumen of humans.
Cases of respiratory depression documented in the literature show that fentanyl was used in bolus form and associated to other medicines of practically unknown pharmacokinetics (8,9).
With the development of new anesthetic agents with well-established pharmacokinetic and pharmacodynamic properties, and with the knowledge about fentanyl´s pharmacokinetic models (10,11,12), these cases of respiratory depressions at the PACUs have practically disappeared, according to the literature.
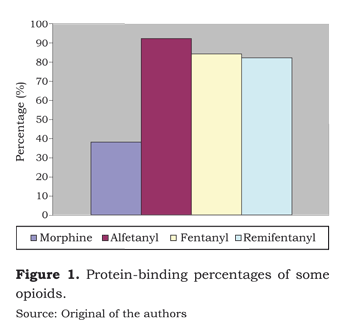
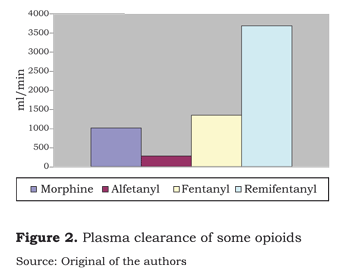
Protein-binding of fentanyl is 84 %, very similar to the binding capacity of alfentanyl and remifentanyl (figure 1). Its distribution volume of 300 to 350 can be accounted for in terms of its large liposolubility and low molecular weight. Its clearance of 1 400 ml per minute is exceeded by remifentanyl (3 700 ml per minute) and is far better than alfentanyl´s clearance (300 ml per minute) (figure 2).
Fentanyl seems to be safer than alfentanyl considering that the potency of the former requires a lower volume for anesthesia than the latter; furthermore, fentanyl´s clearance is higher than alfentanyl (13,14,15).
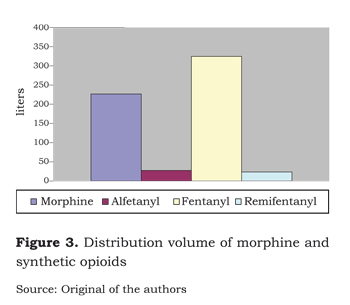
Fentanyl has the highest pKa of all opioids (8,4). Hence, at a pH of 7,4, the percentage of the non-ionized form does not exceed 10 %. Its onset of action then depends on the distribution volume (figure 3).
The average KeO time - defined as the time it takes for the drug to reach half of the plasma concentration in the effector compartment - determines the time to the onset of action.
Fentanyl´s KeO is 0,10 minutes and the mean KeO time is 6,6 minutes. This is due to its high pKa (8,4) that contrary to alfentanyl and remifentanyl (pKa of 6,4 and 7,1) exhibit mean KeO times of 0,6 and 0,9 minutes, respectively (7).
Several models are available for administering fentanyl in a pharmacokinetic manner (8,16). The most widely used are Shafer´s (17,18) and Scott´s (19). The big difference between these two models is the higher distribution volumes used by the Scott´s model. These models are based on populations with a normal BMI; that is to say, they were designed for people with an average body weight of 65 to 70 kg.
The average error in absolute performance varies from 21 % to 32 % for Shafer´s models and from 21 % to 33 % for Scott´s. The mean square error of the former ranges between 34 % and 49 %, while the latter ranges between 27 % and 70 %.
Despite the fact that Shafer´s model has the lowest percentage of error, it is still considered too high; this is why, when using these models, precision is more important than accuracy. So, regardless of the model used, what´s important is to always use the same model in every patient. Furthermore, when reading about fentanyl´s plasma concentrations we most keep in mind the model used in each particular study in order to avoid misinterpretations or program inappropriate infusions.
If a 25-year old man who weights 65 kg and is 1,65 m tall, receives 3 µg/kg of fentanyl, according to Shafer he will reach a plasma peak of 27 ng/ml, while according to Scott it will peak at 13 ng/ml. The effect-site concentration after five minutes will be 3,4 ng/ml and 2,4 ng/ml, respectively (figures 4 & 5).
There are three reasons why we prefer Scott´s model. The first is that some textbooks like Barash´s describe plasma concentrations based on this model. The second reason is that the probability of non-response to the surgical stimulus in 50 % of the patients when using propofol at a plasma concentration of 3,7 µg/ml, is achieved with fentanyl at a plasma concentration of 1,1 ng/ml for 300-minute surgeries, according to Scott´s model. The third reason is that the correlation to administer fentanyl in obese patients, in accordance with the nomogram designed by Shibutani (20) and the administration making a 20 % additional adjustment over the ideal weight, is more consistent with Scott´s model (figure 6).
When administered alone, the plasma concentration of propofol required for not having 50 % of the patients respond to the stimulus during the surgical incision is 15,2 µg/ml (21). When the concentration of fentanyl is 1 ng/ml, there is less need for a propofol concentration of 5 µg/ml, and at concentrations of fentanyl of 2 ng/ml, the need for propofol drops down to 2,5 µg/ml; at concentrations of 3 ng/ml of fentanyl, it drops down to 1,3 µg/ml and at concentrations of 4 ng/ml, the requirement of propofol drops down to 1,2 µg/ml. The maximum reduction in the concentration of propofol is achieved at concentrations of fentanyl between 2 and 3 ng/ml (65 % to 80 %, respectively) (22). Above these concentrations of fentanyl, the benefit is insignificant and a clear peak effect of the opioid is markedly observed when combined with a hypnotic.
It can then be concluded that the optimum plasma concentration of fentanyl for intubating a patient, when accompanied with a hypnotic, ranges 2 and 3 ng/ml at the effect-site. This is achieved with fentanyl doses between 3 and 4 µg/kg of body weight. After 30 minutes of administering these doses, the concentration at the effect-site is 0,9 ng/ml and 1,2 ng/ml and at 40 minutes is 0,7 ng/ml and 0,9 ng/ml, respectively. There is a risk of respiratory pressure at concentrations over 1 ng/ml (23,24).
The required concentration of fentanyl for maintaining the anesthesia depends on the time and type of surgery (1,2). Procedures lasting 1 to 5 hours require a fentanyl concentration between 1,3 and 1,1 ng/ml and the concentration of propofol should be between 3,4 and 3,7 µg/ml, respectively. The time before awakening upon the end of the infusion varies from 12 to 20 minutes.
The awakening time when administering fentanyl with propofol does not depend on the concentrations of the former (3,4). Hence, at propofol concentrations of 1,7 µg/ml at the effect site, awakening occurs regardless of whether the fentanyl concentrations are 0,8, 1,0, 1,4, 2,0 or 3,0 ng/ml. Unfortunately propofol has no analgesic effect and thus in insufficient for maintaining the analgesia during the transoperatory period.
In our opinion, the best hypnotics for the administration of fentanyl are desfluorane and sevofluorane (5), because they result in rapid awakening due to their low blood/gas ratio. Furthermore, their analgesic effect allow for controlling any painful stimulus during the transoperatory (30).
The best surgical indications for using fentanyl are those lasting over two hours and having a higher painful stimulus (spine, orthopedic interventions, bypass surgery and chest among others) and with a known estimated surgical time.
Midazolam at a dose of 30 µg/kg prior to induction, with fentanyl doses of 3 to 4 µg/kg, 3 to 5 minutes before intubation and 5 mg/kg dose of sodium thiopental (divided into two parts: two thirds for the starting dose and one third for the second dose one minute prior to intubation), with 2 ED95 of any muscle relaxant, provides excellent intubation conditions without having to open the vaporizer. After intubation, if the infusion rate is adjusted for a plasma concentration of fentanyl of 1,2 to 1,4 ng/ml with 0,8 to 1 MAC of desfluorane at 0,4 liters, that should be enough for achieving adequate anesthetic conditions for surgeries such as bypass and spine. The average consumption of desfluorane with fentanyl in these types of procedures is 12 ml per hour, while the consumption of remifentanyl is 8 ml per hour. In terms of costs, this combination seems very attractive, plus the benefit of a better postoperatory analgesia and rapid awakening.
The pharmacokinetic administration of fentanyl can be achieved via a computerized infusion system (target controlled infusion) or based on nomograms. For instance, to maintain a plasma concentration between 1,1 and 1,2 ng/ml, we should start with an initial infusion of 8 µg/kg per hour for at least fifteen minutes and then proceed to any adjustments needed in accordance with the nomogram and the time for maintaining a plasma concentration (figure 7). It most be noted however that even when this level is reached, we will not achieve the required plasma concentration for intubation (2 ng/ml). Thus, an additional infusion or a loading dose of fentanyl is needed.
A fentanyl dose between 3 and 4 µg/kg, 3 to 5 minutes prior to intubation and continuing with an infusion of 2,2 µg/kg per hour from the very moment at which the initial dose was administered, provides the necessary conditions both for intubation, as well as for starting the surgery promptly. In this way we get 2 ng/ml and ensure the desired plasma concentration, 1,1 to 1,2 ng/ml, from the start of surgery. Once the infusion is started, adjustments are made every 30 minutes to 1,8, 1,5, 1,3, 1,2, 1,1 and 1,0 µg/kg per hour, to maintain the same plasma level (figure 8).
For ambulatory surgery less than two hours, the recommendation is using a fentanyl bolus as follows: surgeries between 15 and 20 minutes long, 2 µg/kg; 30 to 40 minutes, 3 µg/kg; 60 minutes, 4 µg/kg; 90 minutes, 5 µg/kg and 120 minutes, 6 µg/kg. The concentration at the effect-site after the time established for each dose is less than de 0,8 ng/ml.
Midazolam at a dose of 30 µg/kg prior to induction, a fentanyl bolus in accordance with time of the surgery (diluted and slow) 3 to 5 minutes before intubating, dose of 2 to 3 mg/kg of propofol and 1 to 2 ED (95) of rocuronium, provide excellent intubation conditions with no need to open the vaporizer. Following the induction, if the MAC (Minimum Alveolar Concentration) of 0,8 to 1 of sevofluorane is maintained at 0,8 liters, that should be enough for adequate anesthetic conditions. The patient awakens at a MAC of 0,1 of sevofluorane. For ENT surgeries, the MAC of sevofluorane with fentanyl at these doses is 0,8 to 1, while the MAC with remifentanyl ranges between 0,5 and 0,6.
The incidence of postoperatory nausea and vomiting with the fentanyl and propofol induction technique is no different from remifentanyl and sevofluorane (7). Different studies have established the incidence of postoperatory nausea and vomiting of remifentanyl vs. opioids such as alfenphanyl and fentanyl (8,9,10,11,12,13,14,15,16) . Most of them conclude that the incidence of postoperatory nausea and vomiting does not change significantly with the various opioids.
In a controlled clinical study in 2005, Rama-Maceiras et al (17) showed that the incidence of nausea and vomiting was higher with fentanyl as compared to remifentanyl, in patients who underwent plastic surgery using a hypnotic agent such as propofol. Unfortunately, the protocol in this study did not administer fentanyl in a pharmacokinetic manner and the plasma concentration is unknown. Furthermore, the criteria for administering fentanyl were the same as for remifentanyl, without taking the differences in the pharmacokinetics of the two drugs into account. The average surgical time was 129±79 min (53 to 325) and the amount of fentanyl used was 630±420 µg (13 to 1 920 µg). The analysis of the data does not specify whether the incidence of postoperatory nausea and vomiting was higher in patients requiring a higher dose of fentanyl.
The question remains unanswered, but the available studies for the moment seem to show no differences between fentanyl and remifentanyl in terms of postoperatory nausea and vomiting.
Another adverse event of fentanyl, as of the other opioids (18), particularly when administered at high doses or fast bolus, is the woody chest or muscle stiffness (19). The clinical presentation may range from mild muscle stiffness, ranging from chill-type movements, up to episodes similar to a generalized tonicoclonic seizure (20,21,22). Tension on the laryngeal muscles, particularly the vocal folds - similar to a laryngospasm that can be reverted with naloxine (22) or with a full dose of muscle relaxant - 2 to 3 ED (95) has been documented with regards to these episodes.
The incidence of this syndrome (higher in the elderly) (36,24), occurs at concentrations above 10 ng/ml of fentanyl33 in the effect-site and it has been suggested that it is related to the modulation of the gamma-aminobutiric acid modulation pathway in the spinal cord and in the basal ganglia, through binding of the µ and ? opioid receptors (36). Animal studies also suggest that the stimulation of the opioid receptors in the basal ganglia leads to muscle rigidity. Other authors have documented in rats (25), that the adenosine A1 and A2 receptors in the spinal cord may be more relevant for explaining the muscle rigidity than CNS receptors.
CONCLUSION
fentanyl is an opioid with particular pharmacokinetic and pharmacodynamic characteristics making it ideal for extended surgical procedures requiring considerable analgesia. The concomitant use of drugs affecting the CP450, such as dexametasone, midazolam and antidepressants, inter alia, reduces the production of norfentanyl (inactive metabolite). A dose of 3 to 4 µg/kg for intubation, reaches a plasma concentration below 1 ng/ml (limit for apnea) after 30 minutes. fentanyl administered in accordance with a pharmacokinetic model, adjusting for a plasma concentration between 1,2 and 1,4 ng/ml, provides adequate levels of analgesia, reducing any side effects such as respiratory depression. The ideal hypnotic agent for administering fentanyl in continuous infusion, in surgeries over two hours long, is desfluorane or sevofluorane at concentrations between 0,8 and 1 MAC.
REFERENCES
1. Gutstein HB, Akil H. Opioid Analgesics. En Goodman & Gilman`s. The Pharmacological Basis of Therapeutics. 11th Ed. New York. McGraw Hill; 2006. p. 547-90.
2. Coda B. Opioids. En Barash PG. Clinical Anesthesia. Fifth edition. Philadelphia: Lippincott Williams and Wilkins; 2006. p. 353-83.
3. Wang H, Li EY, Xu GW, Wang CS, Gong YL, Li P. Intravenous fentanyl is exhaled and the concentration fluctuates with time. J Int Med Res. 2009;37(4):1158-66.
4. Coral IS, Moore AR, Strunin L. Plasma concentrations of fentanyl in normal surgical patients with severe renal failure. Br J Anaesth. 1980; 52:101.
5. Fine, P, Portenoy, RK. Opioid analgesia, 2nd Edition. McGraw Hill, New York 2007.
6. Labroo RB, Paine MF, Thummel KE, Kharasch ED. fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab Dispos. 1997;25(9):1072-80.
7. Stoeckel H, Hengstmann JH, Schüttler J. pharmacokinetics of fentanyl as a possible explanation for recurrence of respiratory depression. Br J Anaesth. 1979; 51(8): 741-5.
8. Adams A, Pybus D. Delayed respiratory depression after use of fentanyl during anaesthesia. Br Med J. 1978; 1(6108): 278-9.
9. Williams JH. Delayed respiratory depression after use of fentanyl. Br Medical J. 1978; 1:441.
10. Shafer S, Varvel J. pharmacokinetics, Pharmacodynamics, and Rational Opioid Selection. Anesthesiology. 1991; 74 (1): 53-63.
11. Yassen A, Olofsen E, Romberg R, Sarton E, Teppema L, Danhof M et al. Mechanism-based PK/PD modeling of the respiratory depressant effect of buprenorphine and fentanyl in healthy volunteers. Clin Pharmacol Ther. 2007; 81(1):50-8.
12. Magosso E, Ursino M, van Oostrom JH. Opioid-induced respiratory depression: a mathematical model for fentanyl. IEEE Trans Biomed Eng. 2004;51(7):1115-28.
13. Sternlo JE, Sandin RH. Recurrent respiratory depression after total intravenous anaesthesia with propofol and alfentanil. Anaesthesia. 1998;53(4):378-81.
14. Krane BD, Kreutz JM, Johnson DL, Mazuzan JE Jr. Alfentanil and delayed respiratory depression: case studies and review. Anesth Analg. 1990;70(5):557-61.
15. Scholz J, Steinfath M, Schulz M. Clinical pharmacokinetics of alfentanil, fentanyl and sufentanil. An update. Clin Pharmacokinet. 1996;31(4):275-92.
16. Glass P, Jacobs J, Smith R, Ginsberg B, Quill T, Bai S et al. Pharmacokinetic Model-driven Infusion of fentanyl: Assesment of Accuracy. Anesthesiology. 1990; 73 (6): 1082-90.
17. Shafer S, Varvel J, Aziz N, Scott J. pharmacokinetics of fentanyl Administered by Computer - controlled Infusion Pump. Anesthesiology. 1990; 73 (6): 1091-1102.
18. Youngs E, Shafer S. Pharmacokinetic Parameters Relevant to Recovery from Opioids. Anesthesiology 1994; 81 (4): 833-42.
19. Scott JC, Stanski DR. Decreased fentanyl and alfentanil dose requirements with age. A simultaneous pharmacokinetic and pharmacodynamic evaluation. J Pharmacol Exp Ther. 1987;240(1):159-66.
20. Shibutani K, Inchiosa M, Sawada K, Bairamian M. Accuracy of Pharmacokinetic Models for Predicting Plasma fentanyl Concentrations in Lean and Obese Surgical Patients. Derivation of Dosing Weight ("Pharmacokinetic Mass"). Anesthesiology. 2004; 101(3): 603-13.
21. Vuyk J, Mertens M, Olofsen E, Burm A, Bovill J. Propofol Anesthesia and Rational Opioid Selection: Determination of Optimal EC sub 50 -EC sub 95 Propofol-Opioid Concentrations that Assure Adequate Anesthesia and a Rapid Return of Consciousness. Anesthesiology. 1997; 87 (6): 1549-62.
22. Smith C, McEwan A, Jhaveri R, Wilkinson M, Goodman D, Smith R et al. The Interaction of fentanyl onthe Cp50 of Propofol for Loss of Conciousness and Skin Incision. Anethesiology, 1994; 81(4):820-8.
23. Mildh LH, Scheinin H, Kirvelä OA. The concentration-effect relationship of the respiratory depressant effects of alfentanil and fentanyl. Anesth Analg. 2001;93(4):939-46.
24. Cartwright P, Prys-Roberts C, Gill K, Dye A, Stafford M, Gray A. Ventilatory depression related to plasma fentanyl concentrations during and after anesthesia in humans. Anesth Analg. 1983;62(11):966-74.
25. Kaneda K, Han TH. Comparative population pharmacokinetics of fentanyl using non-linear mixed effect modeling: burns vs. non-burns. Burns. 2009;35(6):790-7.
26. Koehntop DE, Rodman JH. fentanyl pharmacokinetics in patients undergoing renal transplantation. Pharmacotherapy 1997; 17:746-52.
27. Han T, Kim D, Kil H, Inagaki Y. The effects of plasma fentanyl concentrations on propofol requirement, emergence from anesthesia, and postoperative analgesia in propofol-nitrous oxide anesthesia. Anesth Analg. 2000;90(6):1365-71.
28. Iwakiri H, Nagata O, Matsukawa T, Ozaki M, Sessler D. Effect-Site Concentration of Propofol for Recovery of Consciousness Is Virtually Independent of fentanyl Effect-Site Concentration. Anesth Analg 2003;96:1651-55.
29. Ledowski T, Manopas A, Lauer S. Bronchial mucus transport velocity in patients receiving desflurane and fentanyl vs. sevoflurane and fentanyl. Eur J Anaesthesiol. 2008;25(9):752-5.
30. Feld J, Hoffman W, Paisansathan C, Park H, Ananda RC. Autonomic activity during dexmedetomidine or fentanyl infusion with desflurane anesthesia. J Clin Anesth 2007; 19(1): 30-6.
31. Watcha M, White P. Postoperative Nausea and Vomiting Its Etiology, Treatment and Prevention. Anesthesiology 1992; 77 (1):162-84.
32. Yang H, Choi P, McChesney, Buckley N. Induction with sevoflurane-remifentanil is comparable to propofol-fentanyl-rocuronium in PONV after laparoscopic surgery. Can J Anesth 2004; 51(7): 660-7.
33. Phitayakorn P, Melnick BM, Vicine AF 3rd. Comparison of sufentanil and fentanyl infusions for outpatient anaesthesia. Can J Anaesth. 1987; 34: 242-5.
34. Flacke JW, Bloor BC, Kripke BJ,Flacke WE, Warneck CM, Van Etten AP, Comparison of morphine, meperidine, fentanyl and sufentanil in balanced anesthesia: a double blind study. Anesth Analg. 1985; 64: 897-910.
35. Schuttler J, Albrecht S, Breivik H, Osnes S, Prys-Roberts C, Holder K, A comparison of remifentanil and alfentanil in patients undergoing major abdominal surgery. Anaesthesia. 1997; 52: 307-17.
36. Jellish WS, Leonetti JP, Avramov A, Fluder E, Murdoch J. Remifentanil-based anesthesia versus a propofol technique for otologic surgical procedure. Otolaryngol Head Neck Surg. 2000; 122: 222-7.
37. Michalowski P, DershwitzM, Rosow CE, Conlay LA, Chang YC. Total intravenous anesthesia with remifentanil or alfentanil in ambulatory orthopedic surgery carries minimal risk of postoperative nausea and vomiting. Anesthesiology. 1998; 89 (3A): A34.
38. Davis PJ, Finkel JC, Orr RJ. Fazi L, Mulroy JJ, Woelfel SK. A randomised, doble-blind study of remifentanil versus fentanyl for tonsillectomy and adenoidectomy surgery in pediatric ambulatory surgical patients. Anesth Analg 2000; 90: 863-71.
39. Gaszynski T, Strzelczyk J, Gaszynski W. Post-anesthesia Recovery after Infusion of Propofol with Remifentanil or Alfentanil or fentanyl in Morbidly Obese Patients. Obes Surg. 2004; 14: 498-504.
40. Langevin S, Lessard MR, Trépanier CA, Baribault JP. Alfentanil causes less postoperative nausea and vomiting than equipotent doses of fentanyl or sufentanil in outpatients. Anesthesiology. 1999;91(6):1666-73.
41. Bloomfield EL. The incidence of postoperative nausea and vomiting: a retrospective comparison of alfentanil versus sufentanil. Mil Med. 1992;157(2):59-61.
42. Rama-Maceiras P, Ferreira T, Molins N, Sanduende Y, Bautista A, Rey T. Less postoperative nausea and vomiting after propofol + remifentanil versus propofol + fentanyl anaesthesia during plastic surgery. Acta Anaesthesiol Scand. 2005; 49: 305-11.
43. Bowdle A. Adverse Effects of Opioid Agonists and Agonist-Antagonists in Anaesthesia. Drug Saf. 1998; 19 (3): 173-89.
44. Streisand J, Bailey P, LeMaire L, Ashburn M, Tarver S, Varvel J et al. fentanyl-induced rigidity and Unconsciousness in Human Volunteers. Incidence, Duration and Plasma Concentrations. Anesthesiology 1993; 78(4): 629-34.
45. Bowdle T, Rooke G. Postoperative Myoclonus and Rigidity After Anesthesia with Opioids. Anesth Analg 1994;78:783-6.
46. Roy S, Fortier L. [fentanyl-induced rigidity during emergence from general anesthesia potentiated by venlafexine] La rigidité induite par le fentanyl, pendant le retour à la conscience qui suit l´anesthésie générale, est potentialisée par la venlafexine.Can J Anesth. 2003; 50(1): 32-5.
47. Smith NT, Benthuysen JL, Bickford RG, Sanford TJ, Blasco T, Duke PC, Seizures during opioid anesthetic induction-are they opioid-induced rigidity? Anesthesiology 1989;71:85242.
48. Fahnenstich H, Steffan J, Kau N, Bartmann P. fentanyl-induced chest wall rigidity and laryngospasm in preterm and term infants. Crit Care Med. 2000; 28 (3): 836-9.
49. Bailey P, Wilbrink J, Zwanikken P, Pace N, Stanley T. Anesthetic Induction with fentanyl. Anesth Analg. 1985; 64: 48-53.
50. Lui PW. Involvement of spinal adenosine A1 and A2 receptors in fentanyl-induced muscular rigidity in the rat. Neurosci Lett. 1997; 224(3): 189-92.
1. Gutstein HB, Akil H. Opioid Analgesics. En Goodman & Gilman`s. The Pharmacological Basis of Therapeutics. 11th Ed. New York. McGraw Hill; 2006. p. 547-90. [ Links ]
2. Coda B. Opioids. En Barash PG. Clinical Anesthesia. Fifth edition. Philadelphia: Lippincott Williams and Wilkins; 2006. p. 353-83. [ Links ]
3. Wang H, Li EY, Xu GW, Wang CS, Gong YL, Li P. Intravenous fentanyl is exhaled and the concentration fluctuates with time. J Int Med Res. 2009;37(4):1158-66. [ Links ]
4. Coral IS, Moore AR, Strunin L. Plasma concentrations of fentanyl in normal surgical patients with severe renal failure. Br J Anaesth. 1980; 52:101. [ Links ]
5. Fine, P, Portenoy, RK. Opioid analgesia, 2nd Edition. McGraw Hill, New York 2007. [ Links ]
6. Labroo RB, Paine MF, Thummel KE, Kharasch ED. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab Dispos. 1997;25(9):1072-80. [ Links ]
7. Stoeckel H, Hengstmann JH, Schüttler J. Pharmacokinetics of fentanyl as a possible explanation for recurrence of respiratory depression. Br J Anaesth. 1979; 51(8): 741-5. [ Links ]
8. Adams A, Pybus D. Delayed respiratory depression after use of fentanyl during anaesthesia. Br Med J. 1978; 1(6108): 278-9. [ Links ]
9. Williams JH. Delayed respiratory depression after use of fentanyl. Br Medical J. 1978; 1:441. [ Links ]
10. Shafer S, Varvel J. Pharmacokinetics, Pharmacodynamics, and Rational Opioid Selection. Anesthesiology. 1991; 74 (1): 53-63. [ Links ]
11. Yassen A, Olofsen E, Romberg R, Sarton E, Teppema L, Danhof M et al. Mechanism-based PK/PD modeling of the respiratory depressant effect of buprenorphine and fentanyl in healthy volunteers. Clin Pharmacol Ther. 2007; 81(1):50-8. [ Links ]
12. Magosso E, Ursino M, van Oostrom JH. Opioid-induced respiratory depression: a mathematical model for fentanyl. IEEE Trans Biomed Eng. 2004;51(7):1115-28. [ Links ]
13. Sternlo JE, Sandin RH. Recurrent respiratory depression after total intravenous anaesthesia with propofol and alfentanil. Anaesthesia. 1998;53(4):378-81. [ Links ]
14. Krane BD, Kreutz JM, Johnson DL, Mazuzan JE Jr. Alfentanil and delayed respiratory depression: case studies and review. Anesth Analg. 1990;70(5):557-61. [ Links ]
15. Scholz J, Steinfath M, Schulz M. Clinical pharmacokinetics of alfentanil, fentanyl and sufentanil. An update. Clin Pharmacokinet. 1996;31(4):275-92. [ Links ]
16. Glass P, Jacobs J, Smith R, Ginsberg B, Quill T, Bai S et al. Pharmacokinetic Model-driven Infusion of Fentanyl: Assesment of Accuracy. Anesthesiology. 1990; 73 (6): 1082-90. [ Links ]
17. Shafer S, Varvel J, Aziz N, Scott J. Pharmacokinetics of Fentanyl Administered by Computer - controlled Infusion Pump. Anesthesiology. 1990; 73 (6): 1091-1102. [ Links ]
18. Youngs E, Shafer S. Pharmacokinetic Parameters Relevant to Recovery from Opioids. Anesthesiology 1994; 81 (4): 833-42. [ Links ]
19. Scott JC, Stanski DR. Decreased fentanyl and alfentanil dose requirements with age. A simultaneous pharmacokinetic and pharmacodynamic evaluation. J Pharmacol Exp Ther. 1987;240(1):159-66. [ Links ]
20. Shibutani K, Inchiosa M, Sawada K, Bairamian M. Accuracy of Pharmacokinetic Models for Predicting Plasma Fentanyl Concentrations in Lean and Obese Surgical Patients. Derivation of Dosing Weight ("Pharmacokinetic Mass"). Anesthesiology. 2004; 101(3): 603-13. [ Links ]
21. Vuyk J, Mertens M, Olofsen E, Burm A, Bovill J. Propofol Anesthesia and Rational Opioid Selection: Determination of Optimal EC sub 50 -EC sub 95 Propofol-Opioid Concentrations that Assure Adequate Anesthesia and a Rapid Return of Consciousness. Anesthesiology. 1997; 87 (6): 1549-62. [ Links ]
22. Smith C, McEwan A, Jhaveri R, Wilkinson M, Goodman D, Smith R et al. The Interaction of Fentanyl onthe Cp50 of Propofol for Loss of Conciousness and Skin Incision. Anethesiology, 1994; 81(4):820-8. [ Links ]
23. Mildh LH, Scheinin H, Kirvelä OA. The concentration-effect relationship of the respiratory depressant effects of alfentanil and fentanyl. Anesth Analg. 2001;93(4):939-46. [ Links ]
24. Cartwright P, Prys-Roberts C, Gill K, Dye A, Stafford M, Gray A. Ventilatory depression related to plasma fentanyl concentrations during and after anesthesia in humans. Anesth Analg. 1983;62(11):966-74. [ Links ]
25. Kaneda K, Han TH. Comparative population pharmacokinetics of fentanyl using non-linear mixed effect modeling: burns vs. non-burns. Burns. 2009;35(6):790-7. [ Links ]
26. Koehntop DE, Rodman JH. Fentanyl pharmacokinetics in patients undergoing renal transplantation. Pharmacotherapy 1997; 17:746-52. [ Links ]
27. Han T, Kim D, Kil H, Inagaki Y. The effects of plasma fentanyl concentrations on propofol requirement, emergence from anesthesia, and postoperative analgesia in propofol-nitrous oxide anesthesia. Anesth Analg. 2000;90(6):1365-71. [ Links ]
28. Iwakiri H, Nagata O, Matsukawa T, Ozaki M, Sessler D. Effect-Site Concentration of Propofol for Recovery of Consciousness Is Virtually Independent of Fentanyl Effect-Site Concentration. Anesth Analg 2003;96:1651-55. [ Links ]
29. Ledowski T, Manopas A, Lauer S. Bronchial mucus transport velocity in patients receiving desflurane and fentanyl vs. sevoflurane and fentanyl. Eur J Anaesthesiol. 2008;25(9):752-5. [ Links ]
30. Feld J, Hoffman W, Paisansathan C, Park H, Ananda RC. Autonomic activity during dexmedetomidine or fentanyl infusion with desflurane anesthesia. J Clin Anesth 2007; 19(1): 30-6. [ Links ]
31. Watcha M, White P. Postoperative Nausea and Vomiting Its Etiology, Treatment and Prevention. Anesthesiology 1992; 77 (1):162-84. [ Links ]
32. Yang H, Choi P, McChesney, Buckley N. Induction with sevoflurane-remifentanil is comparable to propofol-fentanyl-rocuronium in PONV after laparoscopic surgery. Can J Anesth 2004; 51(7): 660-7. [ Links ]
33. Phitayakorn P, Melnick BM, Vicine AF 3rd. Comparison of sufentanil and fentanyl infusions for outpatient anaesthesia. Can J Anaesth. 1987; 34: 242-5. [ Links ]
34. Flacke JW, Bloor BC, Kripke BJ,Flacke WE, Warneck CM, Van Etten AP, Comparison of morphine, meperidine, fentanyl and sufentanil in balanced anesthesia: a double blind study. Anesth Analg. 1985; 64: 897-910. [ Links ]
35. Schuttler J, Albrecht S, Breivik H, Osnes S, Prys-Roberts C, Holder K, A comparison of remifentanil and alfentanil in patients undergoing major abdominal surgery. Anaesthesia. 1997; 52: 307-17. [ Links ]
36. Jellish WS, Leonetti JP, Avramov A, Fluder E, Murdoch J. Remifentanil-based anesthesia versus a propofol technique for otologic surgical procedure. Otolaryngol Head Neck Surg. 2000; 122: 222-7. [ Links ]
37. Michalowski P, DershwitzM, Rosow CE, Conlay LA, Chang YC. Total intravenous anesthesia with remifentanil or alfentanil in ambulatory orthopedic surgery carries minimal risk of postoperative nausea and vomiting. Anesthesiology. 1998; 89 (3A): A34. [ Links ]
38. Davis PJ, Finkel JC, Orr RJ. Fazi L, Mulroy JJ, Woelfel SK. A randomised, doble-blind study of remifentanil versus fentanyl for tonsillectomy and adenoidectomy surgery in pediatric ambulatory surgical patients. Anesth Analg 2000; 90: 863-71. [ Links ]
39. Gaszynski T, Strzelczyk J, Gaszynski W. Post-anesthesia Recovery after Infusion of Propofol with Remifentanil or Alfentanil or Fentanyl in Morbidly Obese Patients. Obes Surg. 2004; 14: 498-504. [ Links ]
40. Langevin S, Lessard MR, Trépanier CA, Baribault JP. Alfentanil causes less postoperative nausea and vomiting than equipotent doses of fentanyl or sufentanil in outpatients. Anesthesiology. 1999;91(6):1666-73. [ Links ]
41. Bloomfield EL. The incidence of postoperative nausea and vomiting: a retrospective comparison of alfentanil versus sufentanil. Mil Med. 1992;157(2):59-61. [ Links ]
42. Rama-Maceiras P, Ferreira T, Molins N, Sanduende Y, Bautista A, Rey T. Less postoperative nausea and vomiting after propofol + remifentanil versus propofol + fentanyl anaesthesia during plastic surgery. Acta Anaesthesiol Scand. 2005; 49: 305-11. [ Links ]
43. Bowdle A. Adverse Effects of Opioid Agonists and Agonist-Antagonists in Anaesthesia. Drug Saf. 1998; 19 (3): 173-89. [ Links ]
44. Streisand J, Bailey P, LeMaire L, Ashburn M, Tarver S, Varvel J et al. Fentanyl-induced rigidity and Unconsciousness in Human Volunteers. Incidence, Duration and Plasma Concentrations. Anesthesiology 1993; 78(4): 629-34. [ Links ]
45. Bowdle T, Rooke G. Postoperative Myoclonus and Rigidity After Anesthesia with Opioids. Anesth Analg 1994;78:783-6. [ Links ]
46. Roy S, Fortier L. Fentanyl-induced rigidity during emergence from general anesthesia potentiated by venlafexine La rigidité induite par le fentanyl, pendant le retour à la conscience qui suit l´anesthésie générale, est potentialisée par la venlafexine.Can J Anesth. 2003; 50(1): 32-5. [ Links ]
47. Smith NT, Benthuysen JL, Bickford RG, Sanford TJ, Blasco T, Duke PC, Seizures during opioid anesthetic induction-are they opioid-induced rigidity? Anesthesiology 1989;71:85242. [ Links ]
48. Fahnenstich H, Steffan J, Kau N, Bartmann P. Fentanyl-induced chest wall rigidity and laryngospasm in preterm and term infants. Crit Care Med. 2000; 28 (3): 836-9. [ Links ]
49. Bailey P, Wilbrink J, Zwanikken P, Pace N, Stanley T. Anesthetic Induction with Fentanyl. Anesth Analg. 1985; 64: 48-53. [ Links ]
50. Lui PW. Involvement of spinal adenosine A1 and A2 receptors in fentanyl-induced muscular rigidity in the rat. Neurosci Lett. 1997; 224(3): 189-92. [ Links ]











 texto em
texto em