Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Colombian Journal of Anestesiology
versão impressa ISSN 0120-3347
Rev. colomb. anestesiol. vol.43 no.2 Bogotá abr./jun. 2015
Essay
Anesthesia for interventional neuroradiology*
Anestesia en neuroradiología intervencionista
Chanhung Z. Leea,**
a Associate Professor of Anesthesiology, Department of Anesthesia and Perioperative Care, University of California, San Francisco, United States
* Please cite this article as: Lee CZ. Anestesia en neuroradiología intervencionista. Rev Colomb Anestesiol. 2015;43:151-155.
** Corresponding author at: 1001 Potrero Avenue, Building 10, Room 1206, San Francisco, CA 94110, United States. E-mail address: clee4@anesthesia.ucsf.edu
Article info
Article history: Received 5 November 2014 Accepted 6 November 2014 Available online 4 March 2015
Abstract
Interventional Neuroradiology (INR) is firmly established in the management of cerebrovascular diseases. The aim of this manuscript is to present the author's critical review of the literature and interpretation emphasizing perioperative and anesthetic management strategies to prevent complications and minimize their effects if they occur. Planning the anesthetic and perioperative management is predicated on understanding the goals of the therapeutic intervention and anticipating potential problems.
Keywords: Neurosurgical procedures, Hemodynamics, Anesthesia, Endovascular procedures, Central nervous system vascular malformations.
Resumen
La Neuroradiología Intervencionista (NRI) está firmemente establecida en el manejo de la patología cerebrovascular. El objetivo del presente manuscrito es presentar una revisión crítica de la literatura e interpretación por parte del autor, enfatizando las estrategias perioperatorias y anestésicas para prevenir complicaciones y minimizar sus efectos en caso de que estas se presenten. La planeación de la gestión anestésica y perioperatoria se fundamenta en comprender las metas de la intervención terapéutica y anticiparse a los problemas potenciales.
Palabras clave: Procedimientos Neuroquirúrgicos, Hemodinámica, Anestesia, Procedimientos Endovasculares, Malformaciones Vasculares del Sistema Nervioso Central.
Introduction
The aim of this article is to present the author's critical review of the literature and interpretation of the roles of the Anesthesiologist in the management of patients undergoing invasive endovascular procedures to treat vascular diseases, primarily of the central nervous system, emphasizing perioperative and anesthetic management strategies to prevent complications and minimize their effects if they occur. This endovascular practice is usually termed Interventional Neuroradiology (INR) or endovascular neurosurgery. There are several particular anesthetic concerns for INR procedures, including: (1) maintaining immobility during the procedure to facilitate imaging; (2) rapid recovery from anesthesia to facilitate neurological examination and monitoring, or to provide for intermittent evaluation of neurological function during the procedure; (3) managing anticoagulation; (4) managing sudden unexpected procedure-specific complications during the intervention, i.e., hemorrhage or vascular occlusion, which may involve manipulating systemic blood pressures; (5) guiding the medical management of critical care patients during transport to and from the radiology suites; (6) self-protection issues related to radiation safety.1,2
Pre-operative planning and patient preparation
Baseline blood pressure and cardiovascular reserve should be assessed carefully because blood pressure manipulation is commonly required and treatment-related perturbations should be anticipated. A clear sense of the patient's baseline blood pressure range needs to be established. The concordance between blood pressure cuff and intra-arterial readings needs to be considered while decision is usually based on the individual cases. For procedures involving the blood supply to the CNS, beat-to-beat blood pressure monitoring is prudent. Pre-operative blood pressure range is likely to be known through blood pressure cuff values.
Secure intravenous (i.v.) access—with secure connections—should be established with adequate extension tubing to allow drug and fluid administration at maximal distance from the image intensifier during fluoroscopy. Infusions of primary anesthetics or vasoactive agents should be through proximal ports with minimal dead space.
A pulse oximeter probe can be placed on the great toe of the leg that will receive the femoral introducer sheath, and may provide an early warning of femoral artery obstruction or distal thromboembolism, if there is concern about ischemia in high risk patients. Bladder catheters assist in fluid management as well as patient comfort, given that a significant volume of heparinized flush solution and radiographic contrast may be used.
A fundamental knowledge of radiation safety is essential for working in an INR suite. Assume that the X-ray machine is always on. Exposure decreases proportionally to the inverse of the square of the distance from the source of radiation (inverse square law). Digital subtraction angiography (DSA) delivers considerably more radiation than fluoroscopy.
Optimal protection includes use of lead aprons, thyroid shields and radiation exposure badges. A recent study also highlighted the importance of eye-protection for anesthesiologists working significant time in the INR suite.3 Movable lead glass screens may provide additional protection. With proper precautions, the anesthesia team should be exposed to far less than the annual recommended limit for health care workers.
Anesthetic technique
Choice of anesthetic technique
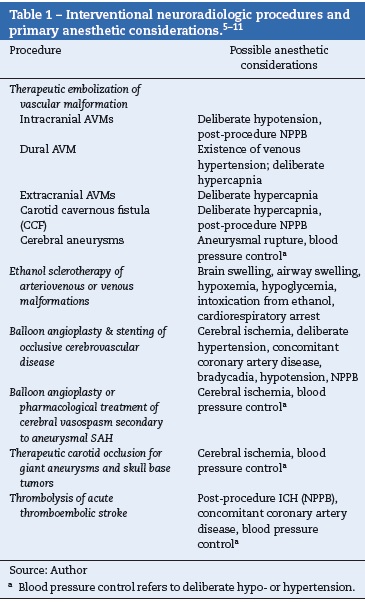
Most centers routinely use general endotracheal anesthesia for complex procedures or those of long duration. There is no clear superior method within well-described considerations for operative neuroanesthesia.4 Specific anesthesia management concerns for common NIR procedures are summarized in Table 1.
General anesthesia
General anesthesia minimizes motion artifacts which improves image quality. Intermittent apnea may be requested by the interventional team to even further reduce motion artifact during DSA. The specific choice of anesthesia may be guided primarily by other cardiac-and cerebrovascular considerations. Total intravenous anesthetic techniques, or a combination of low inspired concentrations of inhalational with intravenous agents may optimize rapid emergence.
Relative normocapnia or slight hypocapnia consistent with the safe conduct of positive pressure ventilation should be maintained unless intracranial pressure is a concern. If a patient has increased intracranial pressure, mild hypocapnia may be indicated prior to apnea during induction and to overcome any vapor-induced vasodilation during maintenance.
Intravenous sedation
One major benefit of intravenous sedation is to allow continual assessment of neurological functions during the procedure. A variety of sedation regimens are available, and specific choices are based on the experience of the practitioner and the goals of anesthetic management. Common to all intravenous sedation techniques is the potential for upper airway obstruction. Placement of nasopharyngeal airways may cause troublesome bleeding in anticoagulated patients and is generally avoided. Dexmedetomidine should be used with care because of the tendency to cause relatively low blood pressure.12
For intravenous sedation cases, careful padding of pressure points and comfortable positioning may decrease the requirement for sedation, anxiolysis and analgesia. Evaluation of the potential ease of laryngoscopy in an emergent situation should take into account access to the airway when imaging is underway.
Anticoagulation
Careful management of coagulation prevents thromboembolic complications during and after the procedure. Generally, after a baseline activated clotting time (ACT) is obtained, intravenous heparin (70 units/kg) is given to a target prolongation of 2-3 times of baseline upon the request from the interventionists, especially if therapeutic procedures are planned. Then heparin often can either be given continuously or as an intermittent bolus with hourly monitoring of ACT as in typical therapeutic regimens.
Heparin-induced thrombocytopenia (HIT) is a rare but important adverse event for heparin anticoagulation. Development of heparin dependent antibodies after initial exposure leads to a prothrombotic syndrome. In high-risk patients, direct thrombin inhibitors can be applied and their effect can be monitored by either aPTT or ACT. Lepirudin and bivalirudin, synthetic derivatives, have half-lives of 40-120 min and about 25 min, respectively. Because these drugs undergo renal elimination, dose adjustments may be needed in patients with renal dysfunction. Argatroban is an alternative agent that undergoes primarily hepatic metabolism.
Antiplatelet agents (aspirin, the glycoprotein IIb/IIIa receptor antagonists and the thienopyridine derivatives) are increasingly being used for cerebrovascular disease management. Abciximab (Rheopro) has been used to treat thromboembolic complications. Abciximab, eptifibatide and tirofiban are glycoprotein IIb/IIIa receptor antagonists. The long duration and potent effect of Abciximab also increase the likelihood of major bleeding. The smaller molecule agents, eptifibatide and tirofiban, are competitive blockers and have a shorter half-life of about 2 h. Thienopyridine derivatives (ticlopidine and clopidogrel) bind to the platelet's ADP receptors and permanently alter the receptor; therefore, the duration of action is the life span of the platelet. The addition of clopidogrel to the antiplatelet regimen is commonly used for procedures that require placement of devices, such as stents, coiling or stent-assisted coiling, primarily in patients that have not had an acute event, such as unruptured aneurysms.
At the end of the procedure or at occurrence of hemorrhagic complication, heparin may be reversed with protamine. Since there is no specific antidote for the direct thrombin inhibitors or the antiplatelet agents, the biological half-life is one of the major considerations in drug choice, and platelet transfusion is a non-specific therapy, should reversal be indicated. There is no currently available accurate test to measure platelet function in patients taking the newer antiplatelet drugs. Desmopressin (DDAVP) has been reported to shorten the prolonged bleeding time of individuals taking antiplatelet agents such as aspirin and ticlopidine. Specific clotting factors, including recombinant factor VIIa and factor IX complex, may be used to rescue severe life-threatening bleeding, including intracranial hemorrhage uncontrolled by standard transfusion therapy. The safety and efficacy of these coagulation factors remain to be investigated.13
Deliberate hypertension
During acute arterial occlusion or vasospasm, a practical way to increase collateral blood flow may be an augmentation of the collateral perfusion pressure by raising the systemic blood pressure. The Circle of Willis is a primary collateral pathway but may be incomplete in as many as more than 20% of otherwise normal subjects. There are also secondary collateral channels that bridge adjacent major vascular territories, most importantly for the long circumferential arteries that supply the hemispheric convexities. These pathways are known as the pial-to-pial collateral or leptomeningeal pathways.
The extent to which the blood pressure has to be raised depends on the condition of the patient and the nature of the disease. Typically, during deliberate hypertension the systemic blood pressure is raised by 30-40% above the baseline in the absence of some direct outcome measure, such as resolution of ischemic symptoms or imaging evidence of improved perfusion. Phenylephrine is usually the first line agent for deliberate hypertension and is titrated to achieve the desired level of blood pressure. The EKG and ST segment monitor should be carefully inspected for signs of myocardial ischemia. Other pressors may be appropriate with excessive reflex bradycardia or baseline chronotropic medication or conduction delays. The effects of catecholamines on the cerebral circulation are not predictable, but in general, all of them are acceptable to achieve the target of adequate systemic pressure in the context of brain injury.
The risk of causing hemorrhage into an ischemic area or rupturing an aneurysm must be weighed against the benefits of improving perfusion, but augmentation of blood pressure in the face of acute cerebral ischemia to promote collateral blood supply is probably protective in most settings. Clear communication with the interventional team is certainly crucial to determine the appropriate blood pressure goal and to monitor the effects of manipulation of cerebral perfusion.
Deliberate hypotension
The two primary indications for induced hypotension are: (1) to test cerebrovascular reserve in patients undergoing carotid occlusion; and (2) to slow flow in a feeding artery of brain arterio-venous malformations before glue injection (sometimes termed "flow arrest").14 The most important factor in choosing a hypotensive agent is the ability to safely and expeditiously achieve the desired reduction in blood pressure while maintaining the patient physiologically stable, and if awake, not interfere with neurological assessment. It is important to realize that the population average lower limit of autoregulation is closer to 70 mmHg than the 50 mmHg frequently shown in text books.15 This may have important implications for use of deliberate hypotension, since ensuring adequate cerebral perfusion is critical for patient safety, especially if general anesthesia is used.
Management of neurological and procedural crises
A well thought-out plan, coupled with rapid and effective communication between the anesthesia and radiology teams, is critical for good outcomes. The primary responsibility of the anesthesia team is to preserve gas exchange and, if needed, secure the airway and support systemic circulation. The anesthesiologist should communicate with the INR team and determine whether the problem is hemorrhagic or occlusive.
In the setting of vascular occlusion, the goal is to increase distal perfusion by blood pressure augmentation with or without direct thrombolysis. If the problem is hemorrhagic, immediate cessation of heparin and reversal with protamine is indicated, after discussion with the interventional team. As an emergency reversal dose, 1 mg protamine can be given for each 100 units of initial heparin dosage that resulted in therapeutic anticoagulation. The ACT can then be used to fine-tune the final protamine dose. Complications of protamine administration include hypotension, true anaphylaxis and pulmonary hypertension. In the anesthetized or comatose patient, the sudden onset of bradycardia and hypertension (Cushing response) or the endovascular therapist's diagnosis of extravasation of contrast may be the only clues to a developing hemorrhage. Most cases of vascular rupture can be managed in the angiography suite. The INR team can attempt to seal the rupture site endovascularly and abort the procedure; a ventriculostomy catheter may be placed emergently in the angiography suite. Patients with suspected rupture will require emergent CT scan, but emergent craniotomy is usually not indicated.
Post-operative management
Endovascular surgery patients pass the immediate postoperative period in a monitored setting such as a high dependency unit or intensive care unit to watch for signs of hemodynamic instability or neurologic deterioration. Control of blood pressure may be necessary during transport and postoperative recovery, e.g., induced hypertension, if indicated. In particular, patients undergoing treatment of extracranial carotid disease are prone to postprocedural hemodynamic instability, similar to post carotid endartectomy patients.16
Abrupt restoration of normal systemic pressure to a chronically hypotensive (ischemic) vascular bed may overwhelm autoregulatory capacity and result in hemorrhage or swelling (normal perfusion pressure breakthrough, NPPB). The mechanism is unclear, but it is probably not simply a hemodynamic effect. Nonetheless, cerebral hyperemia is probably exacerbated by uncontrolled increases in systemic arterial blood pressure. In the absence of collateral perfusion pressure inadequacy, fastidious attention to preventing hypertension is warranted.17
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
None
References
1. Young WL, Pile-Spellman J. Anesthetic considerations for interventional neuroradiology. Anesthesiology. 1994;80:427-56. [ Links ]
2. Young WL, Pile-Spellman J, Hacein-Bey L, Joshi S. Invasive neuroradiologic procedures for cerebrovascular abnormalities: anesthetic considerations. Anesthesiol Clin North Am. 1997;15:631-53. [ Links ]
3. Anastasian ZH, Strozyk D, Meyers PM, Wang S, Berman MF. Radiation exposure of the anesthesiologist in the neurointerventional suite. Anesthesiology. 2011;114:512-20. [ Links ]
4. McDonagh DL, Olson DM, Kalia JS, Gupta R, Abou-Chebl A, Zaidat OO. Anesthesia and sedation practices among neurointerventionalists during acute ischemic stroke endovascular therapy. Front Neurol. 2010;1:118. [ Links ]
5. Feng L, Fitzsimmons BF, Young WL, Berman MF, Lin E, Aagaard BD, Duong H, Pile-Spellman J. Intraarterially administered verapamil as adjunct therapy for cerebral vasospasm: safety and 2-year experience. AJNR Am J Neuroradiol. 2002;23:1284-90. [ Links ]
6. van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. AJNR Am J Neuroradiol. 2007;28:172-7. [ Links ]
7. Linfante I, Wakhloo AK. Brain aneurysms and arteriovenous malformations: advancements and emerging treatments in endovascular embolization. Structure. 2007;38:1411-7. [ Links ]
8. Higashida RT, Meyers PM, Connors JJ, Sacks D, Strother CM, Barr JD, Wojak JC, Duckwiler GR. Intracranial angioplasty & stenting for cerebral atherosclerosis: a position statement of the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, and the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2005;26:2323-7. [ Links ]
9. Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA. 1999;282:2003-11. [ Links ]
10. Lee CZ, Litt L, Hashimoto T, Young WL. Physiologic monitoring and anesthesia considerations in acute ischemic stroke. J Vasc Interv Radiol. 2004;15:S13-9. [ Links ]
11. Davis MJ, Menon BK, Baghirzada LB, Campos-Herrera CR, Goyal M, Hill MD, Archer DP. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116:396-405. [ Links ]
12. Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461-6. [ Links ]
13. Degos V, Westbroek EM, Lawton MT, Hemphill JC, del Zoppo GJ, Young WL. Perioperative management of coagulation in non-traumatic intracerebral hemorrhage. Anesthesiology. 2013;119:218-27. [ Links ] [ Links ]
15. Drummond JC, Patel PM. Neurosurgical anesthesia (chapter 63). In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL, editors. Miller's anesthesia. New York: Churchill Livingstone; 2010. p. 2045-87. [ Links ]
16. Meyers PM, Higashida RT, Phatouros CC, Malek AM, Lempert TE, Dowd CF, Halbach VV. Cerebral hyperperfusion syndrome after percutaneous transluminal stenting of the craniocervical arteries. Neurosurgery. 2000;47:335-43. [ Links ]
17. Phatouros CC, Meyers PM, Higashida RT, Malek AM, Lempert TE, Dowd CF, Halbach VV. Intracranial hemorrhage and cerebral hyperperfusion syndrome after extracranial carotid artery angioplasty and stent placement. AJNR Am J Neuroradiol. 2002;23:503-4. [ Links ]











 texto em
texto em