What do we know about this issue?
No systematic reviews or meta-analyses assessing the hemodynamic changes in patients with the use of low-dose ketamine during the post-operative period were identified.
There is no evidence suggesting that low doses of ketamine are significantly associated with heart rate or blood pressure alterations in patients with acute postoperative pain, treated with this analgesic approach.
What does this study contribute to?
Considering that the cardiovascular effects of ketamine at anesthetic doses are contraindicated for patients with cardiovascular disease, these low-dose ketamine infusion findings contribute with new information allowing to consider its use for a larger population of patients with cardiovascular risk, based on its well-known opioid-sparing effect and analgesia in cases of pain complicated with hyperalgesia/opioid tolerance.
INTRODUCTION
Ketamine is effective for postoperative pain analgesia. Its antagonistic effect on the NMDA receptor reduces central sensitization in the dorsal horn during nociception, in addition to promoting the descending pain inhibition through the monoaminergic pathway. 1,2 The use of low-dose ketamine infusion (LDKI) has increased in postoperative pain models associated with severe pain. 3,4
Ketamine is associated with dose-dependent adverse effects - such as psychomimetic - which according to the various studies published so far apparently do not impact the recovery. 4,5 The cardiovascular effects are usually less frequent, but may be a contraindication for high risk patients.
There is a limited amount ofinformation about the cardiovascular effects, most of which are reported for anesthetic doses of ketamine during the perioperative period. The cardiovascular effects of LDKI are described in the literature mostly with regards to healthy patients, but there is limited information about the impact on high-risk patients, or patients with cardiovascular disease. Cardiopulmonary toxicity is rare in high-dose ketamine, with limited effects on the transient sympathetic activation. 6-8
A recent consensus showed that analgesia is associated with plasma concentrations of 100-200 ng/mL. 1 Individual pharmacokinetic and pharmacodynamic differences are also argued for LDKI. This is mainly due to the bicompartmental model of ketamine which accounts for the poor response to single doses during the postoperative period. 9
There is limited knowledge about the impact of LDKI in the treatment of acute postoperative pain in primary studies on the hemodynamic changes associated with such intervention. This is an important consideration when making decisions involving patients with frequent cardiovascular diseases.
The objective of this study was to conduct a systematic review of clinical trials comparing LDKI versus placebo in the postoperative setting. The idea was to determine whether the use of ketamine in continuous infusion at analgesic doses during the postoperative period generates hemodynamic changes in blood pressure and heart rate during the first 24 hours of initiation of the infusion, as compared against placebo. Additionally, the use of ketamine at analgesic doses was compared against placebo, in terms of the following clinical outcomes: systolic, diastolic and mean arterial blood pressure, heart rate, pain visual analogue scale at 24, 48 and 72-hour intervals, as well as the equivalent use of morphine at 24 hours and the incidence of psychometric symptoms at 24 hours.
METHODS
A systematic literature review was performed, including randomized clinical trials without any language restriction, which assessed treatment with ketamine, or sketamine for the management of postoperative pain, with an infusion > 24 h at sub-anesthetic doses; these trials were published from January 1st, 2077 until December 31st, 2022, including completed and published trials. Studies with pregnant women, non-sub-anesthetic doses of ketamine (>0.3 mg/kg), and duplicate studies were excluded.
Sources of information and search strategies
A search of illegible articles without any language restriction was conducted, from January ist 2007, until December 31 st, 2022 in Cochrane, PubMed, EMBASE, SciELO, and Lilacs, using a combination of controlled terms such as Medical Subject Heading (MeSH), Emtree and free text terms with several English synonyms. The terms used included: "pain, postoperative"; "postoperative pain"; "post-surgical pain"; "acute postoperative pain"; "ketamine"; "esketamine". The strategy used was a high sensitivity strategy (annex). Additionally, a manual and grey literature search was conducted, using the sources from Anesthesiology and Resuscitation-Pain Congresses. Also a search over the last 15 years of international anesthesia congresses and an analysis of the titles of the clinical trials submitted at the various congresses. The following resources were also reviewed: La Referencia - https//www.lareferencia.info/es, RedCol (https://redcol.minciencias.gov.co/vufind/), OpenAIRE (http://explore.openaire.eu/).
Trial selection process
The Zotero bibliography manager was used. The studies were selected in two phases; the first for titles and abstracts selected for illegibility by two independent reviewers and any duplicates were ruled-out. The investigators' agreement was estimated.
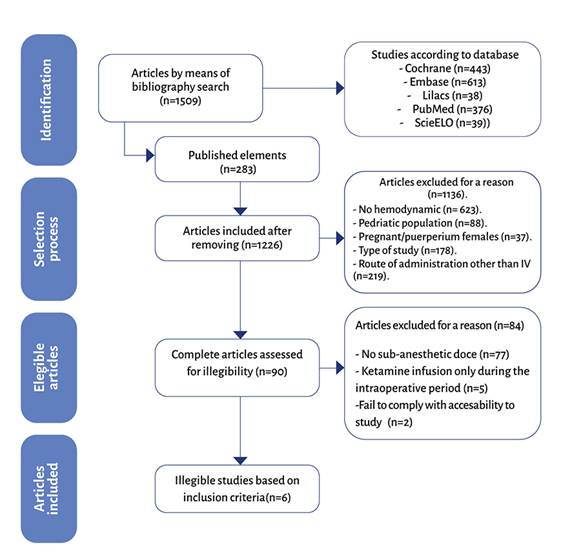
The second phase was a complete reading of the studies included and the ones excluded were listed. Any disagreements arising at these detection levels were settled through a consensus discussion and a third-party review. The reasons for exclusion were illustrated in a studies flowchart (Figure 1).
Process ofextraction and listof data
The process was conducted by two independent investigators and in duplicate using an electronic Excel form, to collect the data from the articles included. The information obtained was then verified with the reviewers. Any disagreements identified at this level were solved through a consensus discussion and/or a third-party review.
The characteristics of the studies assessed were: authors, year of the study, year of publication, journal, study design. The characteristics of the study population included: population size, age, and gender. Intervention: dose, time of dosing, control group. Surgical model. Initial systolic blood pressure (SBP), (mean and standard deviation). Maximum change in SBP (mean and standard deviation); Initial diastolic blood pressure (DBP) (mean and standard deviation), maximum change in DBP (mean and standard deviation); initial heart rate (HR) (mean and standard deviation); maximum change in HR (mean and standard deviation. Time point over the infusion period when the maximum SBP, DBP and HR were observed. Pain assessment using the numerical rating scale (NRS), accumulated use of opioids. Psychomimetic symptoms.
Assessment of risk of bias of individual trials
Two investigators independently assessed the risk of bias of the studies included, using the risk assessment Cochrane collaboration tool (Review Manager version 5.4.1), for randomized clinical trials. Any disagreements arising with regards to the risk of bias or justification thereof, were solved through dialogue until a consensus was reached, with a third author as a referee.
The following domains were evaluated: biases from randomization, biases due to deviations in the interventions planned, biases due to neglected outcomes data, biases in outcomes measurement, biases of selection of published results.
A table of risk of biases was developed for each of the studies included, which was part of the data collection form. Additionally, a risk of bias summary was developed for all the studies using risk figures and traffic-light charts with green if the domain complied with the standards, yellow if the domain was questionable, and red in case of failure to comply.
Effect measurements
For the analysis and synthesis of the relevant outcome variables, different effect measurements were used, according to the type of variable. The quantitative variables, whether continuous or discrete, were analyzed through the differences in standardized measurements (DSM), with their corresponding confidence intervals; in contrast, the quantitative variables were analyzed through the difference of proportions and the RR (Risk Ratio) with its corresponding confidence interval.
Synthesis methods
For the analysis and synthesis of information different statistical models were used, depending on the level of heterogeneity identified in the data of each particular variable subject to analysis. For quantitative, low heterogeneity variables, the differences in means were used, based on fixed effects models; on the contrary, in case of significant heterogeneity, random effect models were developed. For dichotomous qualitative variables, the Mantel-Haenszel (MH) method was used, which involves a fixed effects model to estimate the above-mentioned effect measures for the case.
Assessment of publication bias
The assessment was made in two different ways: a chart through the funnel plot, using the relative risk of each study and the standard error in one of them; the second option used the Egger (linear weighted regression) statistical test considering a statistical bias of publication with a p<0.05.
Quality of evidence evaluation
The evidence was evaluated through each outcome using the system suggested by the Grading of Recommendation Assessment, Development and Evaluation (GRADE) Working Group, and the evidence was classified as high, moderate, low and very low. Several traditional factors of the GRADE system were analyzed: risk of biases, inaccuracy, inconsistency, lack of direct evidence and publication biases. The Guideline Development Tool platform was used in this process.
RESULTS
Selection of studies
A total of 1509 results were identified in the databases, 283 were duplicates and were ruled out, for a total of 1226 publications. The illegibility criteria were reviewed and 1136 were excluded due to lack of hemodynamic data, pregnant women or in immediate puerperium, pediatric patients, route of administration other than IV, and type of study. Of the 90 illegible articles, 84 were excluded due to failure to meet the inclusion criteria - for example, using sub-anesthetic doses of ketamine or administering infusions during the intraoperative period only. Finally, a total of six studies were included in the systematic review (figure 2). The characteristics of the studies selected are illustrated on Table 1. The findings show clinical heterogeneity in the surgical model and methodological heterogeneity which prevent adding the LDKI effect in a meta-analysis for hemodynamic variables in a population of patients with postoperative pain (POP).
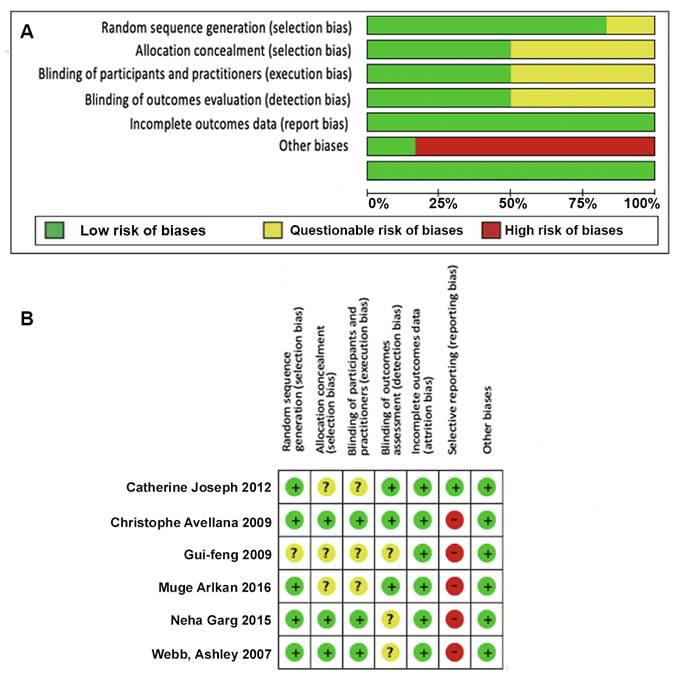
A) Biases throughout the trials. B) Biases for each individual trial. Source: Authors.
Figure 2 Summary of biases of the various trials and biases for each trial depiction.
Table 1 Characteristics of the trials selected for the systematic review.
| Reference | Study type | Intervention | Postoperative infusion dose | Size of the population | Mean age (years) | Surgical model | Result studied |
|---|---|---|---|---|---|---|---|
| Webb et al. 10 | RCT, controlled with saline solution | IV ketamine infusion | 0.1 mg/kg/h in 48 hours | 120 | 63 ± 15 | Major abdominal surgery | SBP, HR |
| Aveline et al. 11 | RCT, controlled with saline solution and nefopam | IV ketamine infusion | 0.06 mg/kg/h in 48 hours | 75 | 72 | Total knee replacement | Presence of tachycardia |
| Deng et al. 12 | RCT, controlled with saline solution salina | IV ketamine infusion | 0.01-0.1 mg/kg/h for 24 hours | 200 | 49 ± 6.1 | Reduction of lower limb fractures | MAP, HR |
| Joseph et al. 13 | RCT, controlled with saline solution salina | IV ketamine infusion | 0.09 mg/kg/h for 48 hours | 60 | 60 | Open thoracotomy | Presence of hypotension |
| Garg et al. 14 | RCT, controlled with saline solution and dexmedetomidine group | IV ketamine infusion | 0.25 mg/kg/h for 24 hours | 66 | 36.45± 13.39 | Spine surgery | PAM, FC, PAS |
| Arikan et al. 15 | RCT controlled with saline solution and magnesium | IV ketamine infusion | 0.05 mg/kg/h for 48 hours | 120 | 59.35 ± 4.96 | Total abdominal hysterectomy | Presence of hypotension and bradycardia |
HR: Heart Rate; MAP: Mean Arterial Pressure presion arterial media; RCT: Randomized Controlled Trial; SBP: Systolic Blood Pressure.
Source: Authors.
Characteristics of the studies
Six randomized clinical trials (RCTs) were included for a total of 641 patients. Webb et al. conducted a RCT with 120 patients undergoing elective laparotomy, administering an intraoperative 0.3 mg/kg bolus and a ketamine infusion of 0.1 mg/kg/h as compared against a normal saline solution control group. All patients received intraoperative tramadol (3 mg/kg), and a tramadol infusion (0.2 mg/kg/h) for 48 h after surgery, and had patient-controlled analgesia available with morphine for rescue analgesia. 10
Aveline et al. conducted a RTC with 75 patients undergoing total knee replacement, comparing a group with a ketamine bolus of 0.2 mg/kg and a 0.12 mg/kg/h intraoperative infusion, followed by postoperative infusion with 0.06 mg/ kg/hour for 48 hours, as compared to the nefopam 60 µg/kg/hour infusion group for 48 hours and a third group with saline solution using the same infusions. 11
Deng et al. conducted an RCT with 200 patients with lower limb fractures undergoing surgery, with 4 groups of intervention: 3 with a 0.5 mg/kg bolus followed by an infusion of 0.1 mg/kg/hour, 0.05 mg/kg/h, 0.01 mg/kg/h for 24 hours and a fourth group receiving an equivalent volume of normal saline solution. Additionally, every group received PCA (patient-controlled analgesia) with a basal infusion of remifentanil and on-demand boluses. The three of ketamine infusion groups were independently compared for the purposes of this review, since they were all independent and at sub-anesthetic doses. 12
Joseph et al. conducted a RCT with 60 patients undergoing thoracotomy. All patients received a thoracic epidural catheter placed before surgery and general anesthesia. Moreover, patients were assigned to two groups to receive ketamine with an initial bolus of 0.5 mg/kg, intraoperative infusion of 0.18 mg/kg/h, and postoperative infusion of ketamine of 0.09 mg/kg/h over 48 hours, or an intravenous placebo. The placebo group received a combination of continuous IV infusion of saline solution and PCA with ropivacaine 1.5 mg/mL during the postoperative period following the thoracotomy. 13
Garg et al. conducted a RCT with 76 patients undergoing spinal surgery, distributed into three groups: the first received a 0.25 mg/kg bolus of ketamine, an infusion of 0.25 mg/kg/h and midazolam bolus of 10 µg/kg, followed by a 10 µg/ kg/h infusion through the same infusion pump. The second group received a bolus of dexmedetomidine of 0.5 µg/kg for 10 minutes, followed by a 0.3 µg/kg/h infusion. The third group received an infusion bolus of saline solution in equivalent volume. 14
Arikan et al. carried out a RCT with 120 female patients undergoing total abdominal hysterectomy assigned to three groups: one group received a ketamine infusion of 0.05 mg/kg/h, the second group a 50 mg/kg magnesium sulphate bolus, followed by a 10 mg/kg/hour infusion; and a third group received saline solution 15.
Risk of bias of individual trials
In five of the trials included in the revision, the patients were randomized into the different groups; however, this could not be established in one trial.
All of the studies provided complete outcome data. 50 % of the trials included met the allocation concealment criteria, blinding of participants/practitioners and outcomes, and in the remaining 50% it was impossible to establish these conditions. 83 % of the trials exhibited reporting bias. Only one trial reported registering the protocol.
Figure 2a shows the summary of biases of the various trials and Figure 2b depicts the biases for each trial.
RESULTS OF THE INDIVIDUAL TRIALS AND SYNTHESIS
Hemodynamic changes in SBP, DBP, MAP, HR at 24 hours
In terms of SBP at 24 hours the study by Webb et al. 10 showed a standardized mean difference (SMD) -1,00 (95 % CI: -7.27 to 5.27) (Figure 3A), which is not considered to be statistically significant and it was impossible to make a heterogeneity analysis due to the lack of availability of trials for comparison purposes. A trial by Garg et al. 14 was identified during the search, but the data were incomplete and hence the means and standard deviation analysis was not performed; however, they claim that no SBP alterations were seen at 24 hours, as compared to the placebo group. None of the patients required rescue medications to maintain hemodynamic stability.
For MAP at 24 hours, Deng et al. 12 showed a SD of 1.40 (95 % CI: -0.64 to 3.44) with no statistically significant difference between ketamine and the control group (Figure 3B). There was a non-representative heterogeneity among the three groups of ketamine, as evidenced by a P value for Chi2 of 0.88 and I2 of 0 %.
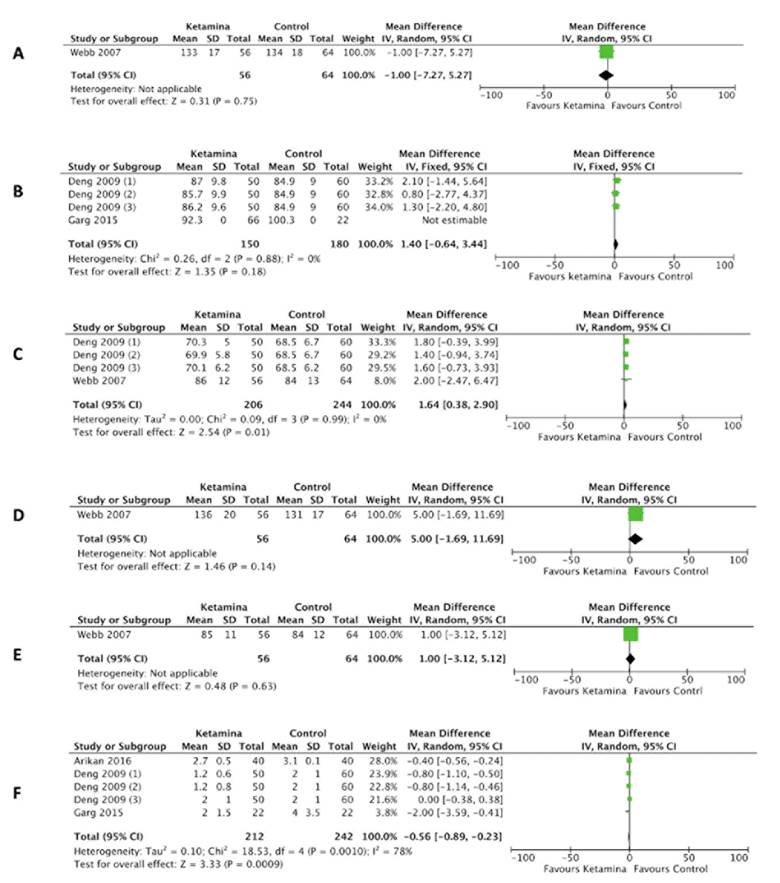
A) Systolic blood pressure at 24 hours. B) Mean arterial pressure at 24 hours. C) Heart Rate at 24 hours. D) Systolic blood pressure at 48 hours. E) Heart rate at 48 hours. F) Pain at 24 hours. Source: Authors.
Figure 3 Forest plot of hemodynamic variables and pain at 24 hours.
In the terms of the results for HR at 24 hours, the SMD was 1.64 (95 % CI: 0.38 to 2.90), which is statistically significant in favor of the control group. 10,12 The heterogeneity was not representative, as evidenced by a P value for ChP of 0.99 and I2 of 0 % (Figure 3C).
SBP, SBP, MAP, HR at 48 hours
In terms of the results for SBP at 48 hours, Webb et al. showed an SMD of 5 (95 % CI: -1.69 to 11.69) (Figure 3D). There is no statistically significant difference and it was impossible to assess the heterogeneity since there were no comparative trials. 10 The search and selection resulted in three trials which contributed with incomplete data and hence could not be included in the statistical analysis; however, at the individual level, Josep et al. described hypotension during the 48 hours following the initiation of the infusion, defined as SBP <80 mm Hg, in 32 % of the population in the ketamine group, against 8 % in the placebo group (p 0.063), which the authors did not consider statistically significant. 13 Arikan et al. reported hypotension in around 7.5 % in the control group and 2.5 % in the magnesium group at 48 hours post-surgery; in contrast, no hypotension developed among the ketamine group. However, the parameters to define this variable were not defined. 15 In the study by Garg et al. no SBP alterations were identified at 48 hours, as compared against the placebo group. None of the patients required any rescue medication to maintain hemodynamic stability. 14
In terms of HR at 48 hours, Webb et al. showed an SMD 1 (95 % CI: -3.1 to 5.12) (figure 3E). There was no statistically significant difference and it was impossible to assess the heterogeneity due to the lack of comparative studies. 10
The search and selection identified two trials which contributed with incomplete data and hence could not be included in the statistical analysis; however, at the individual level, Arikan et al. reported bradycardia of 2.5 % in the magnesium group and 5 % in the control group, 48 hours post-operatively. In contrast, there was no hypotension or bradycardia in the ketamine group. However, the parameters for this variables were not reported. 15 The trial by Aveline et al., reported the presence of tachycardia at 48 hours (HR above 100 beats per minute for 5 continuous minutes) in 4 % of the ketamine group, in 12.5 % in the nefopam group and in 8.3 % in the placebo group; these findings were not statistically significant according to the authors. 11
None of the studies provided DBP data at 24, 48 hours, neither were any data available for SBP, DBP or HR at 72 hours.
Pain at 24 hours after surgery
In terms of outcomes for pain at 24 hours, the combined results of the three trials showed an SMD of -0.56 (95 % CI: -0.89 to -0.23), with statistical significance and a P value for Chi2 of 0.0010 and l2 78 %, with re-presentative heterogeneity which could be explained on the basis of the clinical heterogeneity resulting from the difference in the surgical model, the use of adjuvant medications, the anesthetic technique, different analgesia doses as well as methodological heterogeneity associated with the difference in the size of the population, pain assessment times, concealment of blinding (Figure 3F) 12,14,15. The search and selection showed three studies with incomplete data and hence could not be included in the statistical analysis. Webb et al. and Aveline et al. assessed pain at rest and on movement. This study considered just the pain at rest values. Aveline et al. showed that the VAS scores at rest were significantly lower in patients receiving ketamine as compared to nefopam at 24 hours (p < 0.0001). The study by Webb et al. reported that pain scores (VAS) were lower at rest (P = 0.01, versus on movement (P = 0.02) during 24 hours and the study by Joseph et al., reported a similar accumulated epidural consumption of ropivacaine among the various groups at 24 hours after surgery. The VAS scores were identical at rest, coughing and at 24 hours, with no significant difference. 10,11,13
Postoperative pain at 48 hours
The postoperative 48-hour pain outcome in both studies showed an SMD of -0.38 (95 % CI: -0.73 to -0.02) with statistical significance, with a P value for Chi2 of 0.23 and I2 32 %, with a non-representative heterogeneity (Figure 4A). 14,15
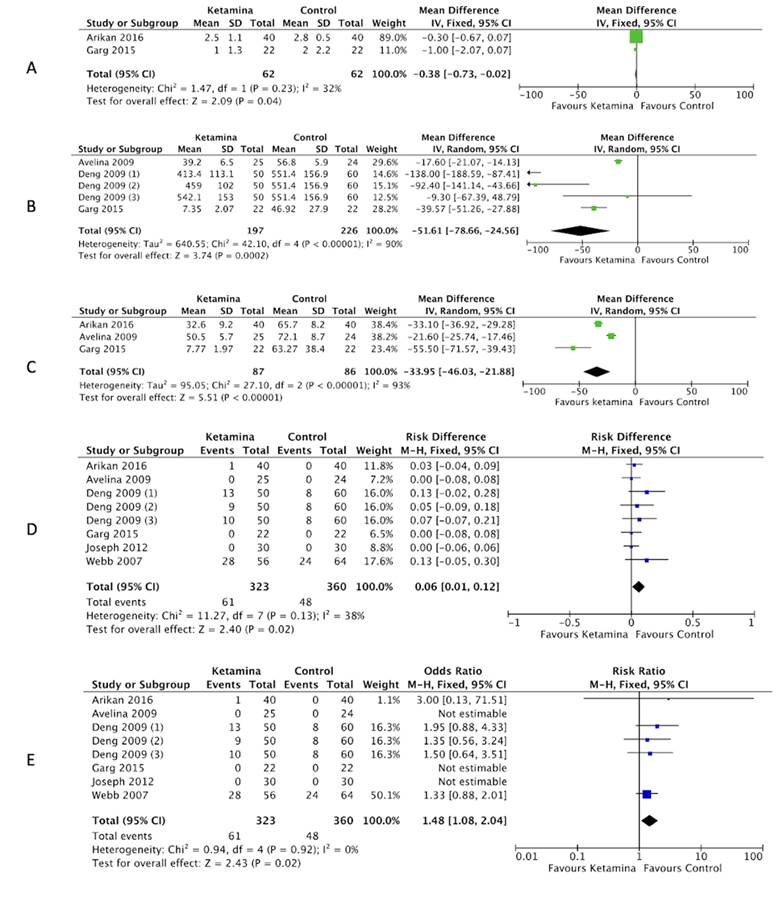
A) Pain at 48 hours. B) Use of morphine at 24 hours. C) Use of morphine at 48 hours. D) Psychometric symptoms (difference of proportions). E) Psychometric symptoms . RR: Relative risk. Source: Authors.
Figure 4 Pain Forest plot, use of opioids and psychometric symptoms.
The search and selection identified three trials which contributed incomplete data and hence should be excluded from the statistical analysis. However, the Aveline et al. study showed that the VAS scores at rest were significantly lower in the patients receiving ketamine as compared to those receiving nefopam at 48 hours (P < 0.0001). 11 The study by Webb et al. established that the VAS scores were lower at rest (P = 0.01) and on movement (P = 0.02) over the first 48 hours 10 and the study by Joseph et al. showed that the accumulated epidural use of ropivacaine was similar among the different groups at 48 hours after surgery. The VAS scores were identical at rest and when coughing at 48 h, with no significant difference. 13 For pain outcomes at 72 hours, the studies failed to contribute with any data.
Use of opioids at 24, 48 and 72 hours
The combined results regarding morphine use at 24 hours of the three trials showed an SMD of -51.61 (95 % CI: -78.66 to -24.56), and hence the use of morphine was considered to decrease in the ketamine groups as compared to the control group, with a statistically significant difference, a representative heterogeneity based on a P value for Chi2 of 0.00001 and I2 90 % (Figure 4B). 11,12,14 The search and selection identified the trial by Webb et al. which was individually analyzed because of incomplete data, but reported that the use of PCA-morphine was 46 % higher in the control group (33.5 mg) versus the ketamine group (23 mg) during the period between 0 to 24 hours (P = 0.003). 10
The analgesia interventions, defined as the need to increase the PCA-morphine bolus or add a basal morphine infusion, were more frequent among the control patients (21 interventions) as compared against the ketamine patients (4 interventions) in the period from 0 to 24 hours (P = 0.01). In terms of morphine use at 48 hours, the SMD was -33.95 (95 % CI: -46.03 to -21.88) evidencing a decline in the morphine requirement in all groups receiving ketamine, as compared against the control group, with a statistical significance. There was a representative heterogeneity expressed by a P value for Chi2 0.00001 and I2 93 % (figure 4C). 11,14,15 The search and selection identified the study by Webb et al., which was individually analyzed because of incomplete data, reporting a use of PCA-morphine of 150 % higher in the control group (30 mg) as compared against the ketamine group (12 mg) during the 24-48 hour period (P = 0.001). The analgesia interventions - defined as the need to increase the PCA-morphine bolus or add a basal morphine infusion over the 24-48 hours period - experienced 9 interventions in control patients and 3 in patients receiving ketamine (P = 0.13). 10 In terms of opioid use at 72 hours, none of the studies reported any data. The trial by Joseph et al. failed to provide specific data on the use of opioids and hence was not included in the analysis. 13
Incidence of psychometric symptoms
In terms of the results for psychometric symptoms, a combination of the six studies included in this review showed a proportions difference of 0.06 (95 % CI: 0.01 to 0.12) and a RR of.48 (95 % CI: 1.08 to 2.04) which indicated that the use of ketamine is a risk factor for the development of such symptoms, with a P for Chi2 0.92 and I2 0 %, showing non-representative heterogeneity (figures 4D and 4E). 10-15
Publication biases
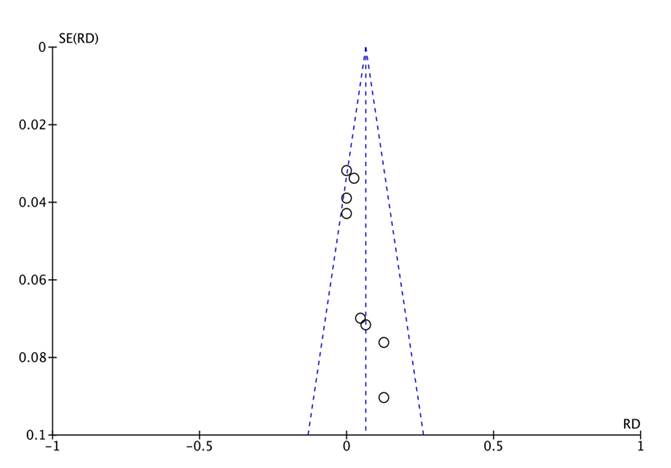
The funnel plot shows symmetry in the distribution of the trials, maintaining the funnel shape and showing in the upper section the studies with the largest sample size; thus, the risk of publication bias is considered low (figure 5). Due to the very small number of trials identified, it was impossible to conduct the Egger test.
The quality of information for each outcome is illustrated in Table 2, with a summary of the certainty of the evidence according to the GRADE system.
Table 2 Quality of the evidence using GRADE for each outcome.
| Certainty assessment | Impact | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N.° of studies | Study design | Risk of bias | Inconsistency | Indirect evidence | Inaccuracy | Other considerations | |||
| Hemodynamic changes (assessing: heart rate, systolic blood pressure, diastolic blood pressure, mean arterial pressure) | |||||||||
| 6 | Randomized trials | Severe a | Severe b | Very severe c | Very severe d | None | In view of the high clinical and statistical heterogeneity present in all the trials, it is impossible to arrive at a common summarized effect to define the hemodynamic changes resulting from the ketamine infusion at analgesic doses, compared against placebo. | ⨁◯◯◯ Very low | Critical |
| Pain at 24, 48 and 72 hours (follow-up: range 24 to 72 hours; measured using the numerical analogue scale) | |||||||||
| 6 | Randomized trials | Severe e | Not severe | Not severe | Severe f | None | Given the high clinical and statistical heterogeneity among all the studies, it is impossible to generalize the effect on postoperative pain of the ketamine infu- sion at analgesic doses as compared to placebo. | ⨁⨁◯◯ Low | Important |
| Use of opioids 24, 48 and 72 hours post-op (follow-up: range 24 to 72 hours; measured with morphine equivalent day) | |||||||||
| 5 | Randomized trials | Severe g | Not severe | Very severe h | Very severe i | None | Given the high clinical and statistical heterogeneity among all the trials, it is impossible to generalize the effect of the use of opioids in patients with ketamine infusion at analgesic doses as compared to placebo. | ⨁◯◯◯ Very low | Important |
| Incidence of psychomimetic symptoms (follow-up: range 24 to 72 hours; measured based on nightmares, terror episodes, delirium, hallucinations or agitation) | |||||||||
| 6 | Randomized trials | Severe j | Very severe k | Very severe l | Not severe | None | Given that the heterogeneity was not representative for this outcome, the ketamine infusion at analgesic doses is considered to have a higher incidence of psychometric symptoms as compared to placebo. | ⨁◯◯◯ Very low | Important |
a. Some of the studies reviewed failed to provide data on hemodynamic changes; additionally, they fail to contribute clear information about the mode of randomization, blinding, and do not specify the protocol registration number.
b. Lack of information about the end-point variables of the study
c. These results were secondary findings in all the studies and it was impossible to collect all the data planned.
d. Due to lack of information about the end-point variables of the study.
e. Some studies reviewed fail to provide pain scales 72 hours after the intervention, Some studies fail to contribute with clear information about the type of randomization, blinding, and do not specify the protocol registration number.
f. Pain scales after 72 hours could not be found and this was one of the objectives of the study.
g. Some of the studies reviewed fail to provide opioid use data at 72 hours, or clear information about the randomization approach, blinding and do not specify the protocol registration number.
h. These results were secondary findings in all the trials; it was impossible to collect all the planned data.
i. Due to lack of data regarding the use of opioids 72 hours after the intervention.
j. Some studies fail to contribute clear information about the randomization approach, blinding, and do not specify the protocol registration number. k. Due to differences in the results of the incidence of psychometric symptoms in the various studies. l. These results were secondary findings in all the studies.
Source: Authors.
DISCUSSION
The purpose of these study was to conduct a systematic review of the hemodynamic effects of ketamine infusion at sub-anesthetic doses (< 0.3 mg/kg/h IV) as adjuvant analgesia for POP pain.
The evidence was found to be low quality, suggestive of a non-association between LDKI and hemodynamic alterations, as compared against the control group. No significant differences were found for blood pressure or heart rate between the LDKI and control groups. One finding to be highlighted is that only two trials showed a lower mean HR in the control group as compared against the LDKI group at 24 hours (SMD 1.64, 95 % CI: 0.38 to 2.90), which was not classified as tachycardia (> 90 beats per minute), and considered of no clinical relevance. 10,12
The descriptive results of the studies assessed did not report any changes in hemodynamics that required treatment, suggesting a relative cardiovascular safety with the use of LDKI, in the surgical models studied. 14
The results of this review are in contrast with the effect of tachycardia and hypertension of bolus doses or doses over 0.5 mg/kg reported in the literature. 7 The idea of LDKI was introduced into the clinical practice over two decades ago. Schmid et al. described the analgesic effect of LDKI in a review including surgical models associated with severe pain. 16 The short half-life of ketamine and the lack of a preventive effect of pain using single preoperative doses, have led to a growing use of LDKI in POP analgesia. Jouguelet et al. report the analgesic effect of LDKI up to 48 hours, in at least eight trials. 2 The author reported outcomes such as lower VAS scores and opioid use, favoring LDKI.
On the other hand, KDLI resulted in lower VAS scores at 24 and 48 hours after surgery. This is consistent with its analgesic effect broadly described in the literature. 3,5,17 Likewise, a reduction in the use of opioids was identified, which could be accounted for by the multimodal strategy in pain control; these findings were already reported in previous studies. 4,18-20 While LDKI has a clear value in the use of opioids and the prevention of opioid-induced tolerance/hyperalgesia, there is very little information about its potential cardiovascular effects at doses lower than the bolus, or those used during the perioperative period. With regards to the psychomimetic symptoms, ketamine at analgesic doses was found to represent a higher risk as compared to placebo, as has been reported in the literature 4; however, this is not a contraindication for its use and should be individually assessed.
Currently, the contraindications for ketamine include uncontrolled cardiovascular disease and liver failure 1; however, the criteria to select LDKI in patients with cardiovascular conditions such as uncontrolled hypertension or coronary heart disease, are not well defined in the literature. In general, the recommendation is to avoid conditions such as tachycardia or hypertension in case of recent onset MI. 21
The results of this study contribute with important information for decision-making regarding the use of LDKI. Changes in the ST segment in previous analyses were observed at single ketamine doses above 0.5 mg/kg. 22 The authors consider that additional trials are needed, including baseline hemodynamic data and different time intervals using LDKI to assess the behavior of such variables in the perioperative setting.
CONCLUSION
This systematic review suggests that LDKI analgesia does not significantly change the hemodynamic behavior of the patients treated for acute POP pain and hence this analgesic option may be considered for patients with cardiovascular risk. The increasing use of LDKI as a strategy in multimodal analgesia warrants further clinical trials to more accurately assess the hemodynamic behavior of LDKI.
Registry and protocol
The Protocol was registered under the Prospero registry (NIHR), with the name: Hemodynamic response to sub-anesthetic doses of ketamine in patients with postoperative pain. Systematic review and meta-analysis. Registration number 411659.
Ethical Responsibilities
The design of this study was a secondary research project in which the unit of analysis is not patients. In accordance with Resolution 8430 of 1993 which establishes the scientific and technical guidelines for research in humans, this research project is free of risk and therefore does not require any informed consent, or approval by the ethics committee for institutional research.











 texto em
texto em